Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis
- PMID: 36090645
- PMCID: PMC9459604
- DOI: 10.21037/tlcr-22-140
Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis
Abstract
Background: Retreatment with immune checkpoint inhibitors (ICIs) might be a subsequent therapeutic option for patients with non-small cell lung cancer (NSCLC) who discontinued initial ICIs treatment because of disease progression, immune-related adverse events (irAEs) or completion of a fixed course, yet little evidence exists on the safety and efficacy of ICIs retreatment to support this strategy.
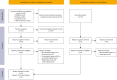
Methods: We searched PubMed, Web of Science, Embase, Cochrane and major meeting libraries for articles about ICIs retreatment in NSCLC for systematic review and meta-analysis. The outcomes included objective response rate (ORR) and disease control rate (DCR) for efficacy and the incidence of all-grade and high-grade irAEs for safety. ICIs rechallenge implies retreatment that can be applied to patients who progressed, while ICIs resumption refers to retreatment for patients who discontinued prior treatment due to an irAE or completion of a fixed course of immunotherapy.
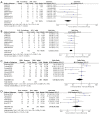
Results: Eighteen studies were enrolled in our analysis. The pooled ORR and DCR of ICIs retreatment were respectively 20% and 54%. ICIs retreatment was associated with a decrease in ORR and DCR compared to prior ICIs treatment (ORR: OR, 0.29, 95% CI: 0.14, 0.63, P=0.002; DCR: OR, 0.53, 95% CI: 0.28-0.99, P=0.05). The pooled ORR and DCR of ICIs rechallenge were 8% and 39%. ICIs rechallenge showed a lower ORR compared with initial ICIs treatment (P<0.05). ICIs resumption presented an ORR of 34% and a DCR of 71%, showing no significant difference in ORR and DCR compared with initial ICIs treatment (P>0.05). Retreated with the same type of ICIs as before showed no difference in ORR and DCR (P>0.05), while with different ICIs was associated with a decrease in ORR and DCR in contrast to initial treatment (P<0.05). The pooled incidence of all-grade and high-grade irAEs after ICIs retreatment in patients with NSCLC were separately 41% and 13% which showed a similar incidence compared with initial ICIs treatment (P>0.05).
Discussion: Retreatment with ICIs is feasible for patients with NSCLC in consideration of its encouraging efficacy and tolerable safety, especially in resumption with ICIs. When it comes to ICIs rechallenge, it is necessary to accurately identify the potential targeted beneficiary population. More large-scale prospective studies are warranted to confirm our discoveries. More attention could be paid to further exploring the efficacy and safety of retreatment concurrently with ICIs and chemotherapy.
Keywords: Non-small cell lung cancer (NSCLC); immune checkpoint inhibitors (ICIs); rechallenge; resumption; retreatment; safety and efficacy.
2022 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-140/coif). YS serves as an Editor-in-Chief of Translational Lung Cancer Research from September 2014 to August 2022. TL serves as an unpaid Associate Editor-in-Chief of Translational Lung Cancer Research. The other authors have no conflicts of interest to declare.
Figures

Comment in
-
Re-administration of immune checkpoint inhibitors for patients with non-small cell lung cancer.Transl Lung Cancer Res. 2022 Nov;11(11):2170-2174. doi: 10.21037/tlcr-22-717. Transl Lung Cancer Res. 2022. PMID: 36519026 Free PMC article. No abstract available.
Similar articles
-
Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review.Ther Adv Med Oncol. 2020 Nov 27;12:1758835920975353. doi: 10.1177/1758835920975353. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 33294036 Free PMC article. Review.
-
Safety and Efficacy of the Rechallenge of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer: A Systemic Review and Meta-Analysis.Front Immunol. 2021 Sep 27;12:730320. doi: 10.3389/fimmu.2021.730320. eCollection 2021. Front Immunol. 2021. PMID: 34646270 Free PMC article.
-
Safety and Efficacy of Rechallenge With Immune Checkpoint Inhibitors in Advanced Solid Tumor: A Systematic Review and Meta-Analysis.Cancer Med. 2024 Oct;13(20):e70324. doi: 10.1002/cam4.70324. Cancer Med. 2024. PMID: 39463070 Free PMC article. Review.
-
Heterogeneous Outcomes of Immune Checkpoint Inhibitor Rechallenge in Patients With NSCLC: A Systematic Review and Meta-Analysis.JTO Clin Res Rep. 2022 Mar 19;3(4):100309. doi: 10.1016/j.jtocrr.2022.100309. eCollection 2022 Apr. JTO Clin Res Rep. 2022. PMID: 35434666 Free PMC article.
-
Safety, efficacy, and survival outcomes of immune checkpoint inhibitors rechallenge in patients with cancer: a systematic review and meta-analysis.Oncologist. 2024 Nov 4;29(11):e1425-e1434. doi: 10.1093/oncolo/oyae134. Oncologist. 2024. PMID: 38940446 Free PMC article.
Cited by
-
Re-administration of immune checkpoint inhibitors for patients with non-small cell lung cancer.Transl Lung Cancer Res. 2022 Nov;11(11):2170-2174. doi: 10.21037/tlcr-22-717. Transl Lung Cancer Res. 2022. PMID: 36519026 Free PMC article. No abstract available.
-
Efficacy of NSCLC Rechallenge with Immune Checkpoint Inhibitors following Disease Progression or Relapse.Cancers (Basel). 2024 Mar 18;16(6):1196. doi: 10.3390/cancers16061196. Cancers (Basel). 2024. PMID: 38539530 Free PMC article. Review.
-
Safety and efficacy of restarting immune checkpoint inhibitors in non-small cell lung cancer patients following immune-related adverse events: a systematic review and meta-analysis.Clin Transl Oncol. 2025 Jan;27(1):196-203. doi: 10.1007/s12094-024-03529-x. Epub 2024 Jun 26. Clin Transl Oncol. 2025. PMID: 38922538 Review.
-
Safety and efficacy of immune checkpoint inhibitor rechallenge in advanced non-small cell lung cancer: a retrospective study.Sci Rep. 2024 Jan 28;14(1):2315. doi: 10.1038/s41598-024-52034-2. Sci Rep. 2024. PMID: 38281979 Free PMC article.
-
Treatment Patterns and Clinical Outcomes Among Patients with Metastatic Non-small Cell Lung Cancer Without Actionable Genomic Alterations Previously Treated with Platinum-Based Chemotherapy and Immunotherapy.Drugs Real World Outcomes. 2024 Sep;11(3):425-439. doi: 10.1007/s40801-024-00440-3. Epub 2024 Jun 19. Drugs Real World Outcomes. 2024. PMID: 38896198 Free PMC article.
References
LinkOut - more resources
Full Text Sources
