Anti-amyloidogenic property of gold nanoparticle decorated quercetin polymer nanorods in pH and temperature induced aggregation of lysozyme
- PMID: 36090438
- PMCID: PMC9389553
- DOI: 10.1039/d2ra03121c
Anti-amyloidogenic property of gold nanoparticle decorated quercetin polymer nanorods in pH and temperature induced aggregation of lysozyme
Abstract
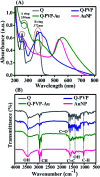
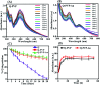
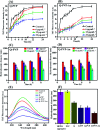
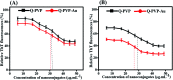
Quercetin is an abundant plant polyphenol effective against several diseases due to its antioxidant and anti-inflammatory activity. Herein, we report novel polymeric quercetin nanorods and the former decorated with gold nanoparticles for the first time. The prepared conjugates quercetin-polyvinylpyrrolidone (Q-PVP) and quercetin-polyvinylpyrrolidone-gold nanoparticles (Q-PVP-Au) were characterized by UV-visible spectroscopy, Fourier transform infrared, dynamic light scattering, and zeta potential measurements. The surface morphology of conjugates was analyzed by field emission scanning electron microscopy. These conjugates exhibit harmonized rod-like morphology with a narrow size distribution. Furthermore, the quercetin conjugates with nanorod morphology exhibited enhanced and prolonged drug release over a long period. The synthesized conjugates were investigated for lysozyme aggregation kinetics. ThT binding assay, fibril size measurement, and electron microscopy results revealed that conjugates could suppress fibrillogenesis in lysozyme. The highest amyloid aggregation inhibition activity (IC50) was obtained against Q-PVP and Q-PVP-Au at 32 μg mL-1 and 30 μg mL-1 respectively. The amyloid aggregate disintegration activity (DC50) obtained against Q-PVP and Q-PVP-Au was 27 μg mL-1 and 29 μg mL-1 respectively. The present quercetin conjugates exhibit enhanced bioavailability and stability. They were potent inhibitors of lysozyme aggregation that may find applications as a therapeutic agent in neurological diseases like Alzheimer's and Parkinson's.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Polymeric curcumin nanospheres for lysozyme aggregation inhibition, antibacterial, and wound healing applications.Environ Sci Pollut Res Int. 2024 Jul;31(34):46625-46640. doi: 10.1007/s11356-023-29160-x. Epub 2023 Sep 9. Environ Sci Pollut Res Int. 2024. PMID: 37688693
-
Functionalisation of Polyvinylpyrrolidone on Gold Nanoparticles Enhances Its Anti-Amyloidogenic Propensity towards Hen Egg White Lysozyme.Biomedicines. 2017 May 3;5(2):19. doi: 10.3390/biomedicines5020019. Biomedicines. 2017. PMID: 28536362 Free PMC article.
-
Insights into the remarkable attenuation of hen egg white lysozyme amyloid fibril formation mediated by biogenic gold nanoparticles stabilized by quercetin-functionalized tara gum.Int J Biol Macromol. 2023 Mar 31;232:123044. doi: 10.1016/j.ijbiomac.2022.12.263. Epub 2022 Dec 29. Int J Biol Macromol. 2023. PMID: 36586653
-
Proline functionalized gold nanoparticles modulates lysozyme fibrillation.Colloids Surf B Biointerfaces. 2019 Feb 1;174:401-408. doi: 10.1016/j.colsurfb.2018.11.032. Epub 2018 Nov 20. Colloids Surf B Biointerfaces. 2019. PMID: 30476794
-
Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications.Biomed Pharmacother. 2017 Aug;92:479-490. doi: 10.1016/j.biopha.2017.05.076. Epub 2017 May 29. Biomed Pharmacother. 2017. PMID: 28570982
Cited by
-
Preparation of caffeic acid grafted chitosan self-assembled micelles to enhance oral bioavailability and antibacterial activity of quercetin.Front Vet Sci. 2023 Jul 5;10:1218025. doi: 10.3389/fvets.2023.1218025. eCollection 2023. Front Vet Sci. 2023. PMID: 37476826 Free PMC article.
-
Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer's Disease.Biology (Basel). 2023 Nov 20;12(11):1453. doi: 10.3390/biology12111453. Biology (Basel). 2023. PMID: 37998052 Free PMC article. Review.
-
Curcumin-Sodium Alginate and Curcumin-Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour.Molecules. 2023 Aug 5;28(15):5893. doi: 10.3390/molecules28155893. Molecules. 2023. PMID: 37570862 Free PMC article.
-
pH-Dependent HEWL-AuNPs Interactions: Optical Study.Molecules. 2023 Dec 22;29(1):82. doi: 10.3390/molecules29010082. Molecules. 2023. PMID: 38202662 Free PMC article.
References
-
- Iadanza M. G. Jackson M. P. Hewitt E. W. Ranson N. A. Radford S. E. Nat. Rev. Mol. Cell Biol. 2018;19:755–773. - PubMed
-
- Paul A. Viswanathan G. K. Mahapatra S. Balboni G. Pacifico S. Gazit E. Segal D. ACS Chem. Neurosci. 2019;10:3510–3520. - PubMed
-
- Swaminathan R., Ravi V. K., Kumar S., Kumar M. V. S. and Chandra N., in Advances in Protein Chemistry and Structural Biology, ed. R. Donev, Academic Press, 2011, vol. 84, pp. 63–111 - PubMed
-
- Ghosh D. Mehra S. Sahay S. Singh P. K. Maji S. K. Int. J. Biol. Macromol. 2017;100:37–54. - PubMed
LinkOut - more resources
Full Text Sources

