Total neoadjuvant therapy or standard chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis
- PMID: 36090336
- PMCID: PMC9458916
- DOI: 10.3389/fsurg.2022.911538
Total neoadjuvant therapy or standard chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis
Abstract
Background and aim: The effectiveness of total neoadjuvant therapy (TNT) on patients with locally advanced rectal cancer (LARC) is controversy. This study aims to compare the prognostic value of TNT with standard neoadjuvant chemoradiotherapy (CRT) for LARC.
Methods: We searched databases (Embase [Ovid], Medline [Ovid], PubMed, Cochrane Library, and Web of Science) for articles published between January 1, 2000, and March 10, 2022. Studies on evaluating the effects of TNT and standard CRT on the prognosis of LARC were included. The primary outcomes were overall survival (OS) and disease-free survival (DFS).
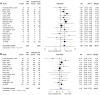
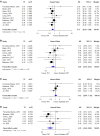
Results: 19 primary studies, involving 10 randomized controlled trials, 3 prospective studies and 6 retrospective studies, with data on 5,074 patients treated for LARC were included in the meta-analysis. Statistical analyses revealed that, compared with standard CRT, TNT significantly improved OS (hazard ratio [HR]=0.77, 95% confidence interval [CI]=0.65-0.90, I 2 = 30%, P = 0.17), DFS (HR = 0.85, 95% CI = 0.74-0.97, I² = 11%, P = 0.35), distant metastases-free survival (DMFS, HR = 0.76, 95% CI = 0.65-0.90, I² = 0%, P = 0.50), pathological complete response rate (pCR, OR = 1.89, 95% CI = 1.61-2.22, I² = 0%, P = 0.47), and R0 resection rate (OR = 1.33, 95% CI = 1.07-1.67, I² = 16%, P = 0.28), but local recurrence-free survival (LRFS, HR = 1.12, 95% CI = 0.90-1.39, I² = 4%, P = 0.37).
Conclusions: Comprehensive literature research shows that TNT showed excellent short-term efficacy in terms of pCR and R0 resection rate while also improved the long-term outcomes of OS, DFS and DMFS, might become a new standard of treatment in patients with LARC. Even so, more studies and longer follow-up were still warranted.
Keywords: locally advanced rectal cancer; meta-analysis; prognosis; standard chemoradiotherapy; total neoadjuvant therapy.
© 2022 Ma, Tan, Liu and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis.Oncologist. 2021 Sep;26(9):e1555-e1566. doi: 10.1002/onco.13824. Epub 2021 Jun 7. Oncologist. 2021. PMID: 33987952 Free PMC article.
-
Efficacy and safety of total neoadjuvant therapy in locally advanced rectal cancer: a meta-analysis.Int J Colorectal Dis. 2023 Apr 1;38(1):89. doi: 10.1007/s00384-023-04376-y. Int J Colorectal Dis. 2023. PMID: 37004572 Review.
-
Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials.Cancers (Basel). 2020 Dec 5;12(12):3655. doi: 10.3390/cancers12123655. Cancers (Basel). 2020. PMID: 33291454 Free PMC article.
-
Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Dec 1;3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097. JAMA Netw Open. 2020. PMID: 33326026 Free PMC article.
-
A Meta-analysis of Total Neoadjuvant Therapies Combining Chemoradiotherapy with Induction or Consolidated Chemotherapy for Locally Advanced Rectal Cancer.J Gastrointest Cancer. 2023 Sep;54(3):693-702. doi: 10.1007/s12029-022-00864-6. Epub 2022 Oct 15. J Gastrointest Cancer. 2023. PMID: 36243897 Review.
Cited by
-
Selecting a TNT Schedule in Locally Advanced Rectal Cancer: Can We Predict Who Actually Benefits?Cancers (Basel). 2023 Apr 30;15(9):2567. doi: 10.3390/cancers15092567. Cancers (Basel). 2023. PMID: 37174033 Free PMC article. Review.
-
MRI-based pre-Radiomics and delta-Radiomics models accurately predict the post-treatment response of rectal adenocarcinoma to neoadjuvant chemoradiotherapy.Front Oncol. 2023 Feb 22;13:1133008. doi: 10.3389/fonc.2023.1133008. eCollection 2023. Front Oncol. 2023. PMID: 36925913 Free PMC article.
-
Integrated Intensified Chemoradiation in the Setting of Total Neoadjuvant Therapy (TNT) in Patients with Locally Advanced Rectal Cancer: A Retrospective Single-Arm Study on Feasibility and Efficacy.Cancers (Basel). 2023 Feb 1;15(3):921. doi: 10.3390/cancers15030921. Cancers (Basel). 2023. PMID: 36765878 Free PMC article.
-
Radiomics Approaches for the Prediction of Pathological Complete Response after Neoadjuvant Treatment in Locally Advanced Rectal Cancer: Ready for Prime Time?Cancers (Basel). 2023 Jan 9;15(2):432. doi: 10.3390/cancers15020432. Cancers (Basel). 2023. PMID: 36672381 Free PMC article. Review.
-
Multi-parametric MRI radiomics for predicting response to neoadjuvant therapy in patients with locally advanced rectal cancer.Jpn J Radiol. 2024 Dec;42(12):1448-1457. doi: 10.1007/s11604-024-01630-3. Epub 2024 Jul 29. Jpn J Radiol. 2024. PMID: 39073521
References
-
- Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. (2017) 18(3):336–46. 10.1016/S1470-2045(17)30086-4 - DOI - PubMed
-
- van Gijn W, Marijnen C, Nagtegaal I, Kranenbarg E, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. (2011) 12(6):575–82. 10.1016/S1470-2045(11)70097-3 - DOI - PubMed
-
- Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. (2009) 373(9666):811–20. 10.1016/S0140-6736(09)60484-0 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

