A simple nanobody-based competitive ELISA to detect antibodies against African swine fever virus
- PMID: 36089216
- PMCID: PMC9797394
- DOI: 10.1016/j.virs.2022.09.004
A simple nanobody-based competitive ELISA to detect antibodies against African swine fever virus
Abstract
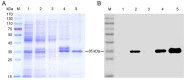
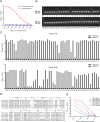
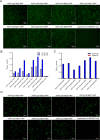
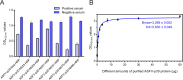
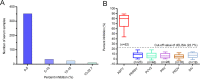
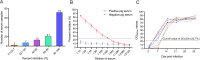
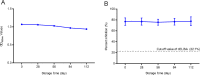
African swine fever virus (ASFV) infection is a big threat to the global pig industry. Because there is no effective vaccine, rapid, low-cost, and simple diagnosis methods are necessary to detect the ASFV infection in pig herds. Nanobodies, with advantages of small molecular weight and easy genetic engineering, have been universally used as reagents for developing diagnostic kits. In this study, the recombinant ASFV-p30 was expressed and served as an antigen to immunize the Bactrian camel. Then, seven nanobodies against ASFV-p30 were screened using phage display technique. Subsequently, the seven nanobodies fused horseradish peroxidase (nanobody-HRP) were secretory expressed and one fusion protein ASFV-p30-Nb75-HRP was selected with the highest sensitivity in blocking ELISA. Using the ASFV-p30-Nb75-HRP fusion protein as a probe, a competitive ELISA (cELISA) was developed for detecting anti-ASFV antibodies in pig sera. The cut-off value of cELISA was determined to be 22.7% by testing 360 negative pig sera. The detection limit of the cELISA for positive pig sera was 1:320, and there was no cross-reaction with anti-other swine virus antibodies. The comparative assay showed that the agreement of the cELISA with a commercial ELISA kit was 100%. More importantly, the developed cELISA showed low cost and easy production as a commercial kit candidate. Collectively, a simple nanobody-based cELISA for detecting antibodies against ASFV is developed and it provides a new method for monitoring ASFV infection in the pig herds.
Keywords: ASFV-p30; African swine fever virus (ASFV); Competitive ELISA; Nanobody; Nanobody-HRP fusion Protein.
Copyright © 2022 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
HRP-conjugated-nanobody-based cELISA for rapid and sensitive clinical detection of ASFV antibodies.Appl Microbiol Biotechnol. 2022 Jun;106(11):4269-4285. doi: 10.1007/s00253-022-11981-4. Epub 2022 May 25. Appl Microbiol Biotechnol. 2022. PMID: 35612629 Free PMC article.
-
K205R specific nanobody-horseradish peroxidase fusions as reagents of competitive ELISA to detect African swine fever virus serum antibodies.BMC Vet Res. 2022 Aug 20;18(1):321. doi: 10.1186/s12917-022-03423-0. BMC Vet Res. 2022. PMID: 35987654 Free PMC article.
-
Nanobodies against African swine fever virus p72 and CD2v proteins as reagents for developing two cELISAs to detect viral antibodies.Virol Sin. 2024 Jun;39(3):478-489. doi: 10.1016/j.virs.2024.04.002. Epub 2024 Apr 6. Virol Sin. 2024. PMID: 38588947 Free PMC article.
-
Development of a Nanobody-Based Competitive Enzyme-Linked Immunosorbent Assay for Efficiently and Specifically Detecting Antibodies against Genotype 2 Porcine Reproductive and Respiratory Syndrome Viruses.J Clin Microbiol. 2021 Nov 18;59(12):e0158021. doi: 10.1128/JCM.01580-21. Epub 2021 Sep 15. J Clin Microbiol. 2021. PMID: 34524888 Free PMC article.
-
African swine fever virus serodiagnosis: a general review with a focus on the analyses of African serum samples.Virus Res. 2013 Apr;173(1):159-67. doi: 10.1016/j.virusres.2012.10.021. Epub 2012 Nov 3. Virus Res. 2013. PMID: 23131491 Review.
Cited by
-
SNX32 is a host restriction factor that degrades African swine fever virus CP204L via the RAB1B-dependent autophagy pathway.J Virol. 2024 Jan 23;98(1):e0159923. doi: 10.1128/jvi.01599-23. Epub 2024 Jan 3. J Virol. 2024. PMID: 38169281 Free PMC article.
-
A review on camelid nanobodies with potential application in veterinary medicine.Vet Res Commun. 2024 Aug;48(4):2051-2068. doi: 10.1007/s11259-024-10432-x. Epub 2024 Jun 13. Vet Res Commun. 2024. PMID: 38869749 Review.
-
Development of a Phage-Displayed Nanobody-Based Competitive Immunoassay for the Sensitive Detection of Soybean Agglutinin.Foods. 2024 Jun 16;13(12):1893. doi: 10.3390/foods13121893. Foods. 2024. PMID: 38928834 Free PMC article.
-
Development of an enzyme-linked immunosorbent assay based on viral antigen capture by anti-spike glycoprotein monoclonal antibody for detecting immunoglobulin A antibodies against porcine epidemic diarrhea virus in milk.BMC Vet Res. 2023 Feb 11;19(1):46. doi: 10.1186/s12917-023-03605-4. BMC Vet Res. 2023. PMID: 36765329 Free PMC article.
-
A Novel Blocking Enzyme-Linked Immunosorbent Assay Based on a Biotinylated Nanobody for the Rapid and Sensitive Clinical Detection of Classical Swine Fever Virus Antibodies.Microbiol Spectr. 2023 Feb 14;11(1):e0299622. doi: 10.1128/spectrum.02996-22. Epub 2023 Jan 23. Microbiol Spectr. 2023. PMID: 36688674 Free PMC article.
References
-
- Afonso C.L., Alcaraz C., Brun A., Sussman M.D., Onisk D.V., Escribano J.M., Rock D.L. Characterization of p30, a highly antigenic membrane and secreted protein of african swine fever virus. Virology. 1992;189:368–373. - PubMed
-
- Barderas M.G., Wigdorovitz A., Merelo F., Beitia F., Alonso C., Borca M.V., Escribano J.M. Serodiagnosis of african swine fever using the recombinant protein p30 expressed in insect larvae. J. Virol. Methods. 2000;89:129–136. - PubMed
-
- Barthelemy P.A., Raab H., Appleton B.A., Bond C.J., Wu P., Wiesmann C., Sidhu S.S. Comprehensive analysis of the factors contributing to the stability and solubility of autonomous human vh domains. J. Biol. Chem. 2008;283:3639–3654. - PubMed
-
- Blome S., Gabriel C., Beer M. Pathogenesis of african swine fever in domestic pigs and european wild boar. Virus Res. 2013;173:122–130. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

