Space microgravity improves proliferation of human iPSC-derived cardiomyocytes
- PMID: 36084640
- PMCID: PMC9561632
- DOI: 10.1016/j.stemcr.2022.08.007
Space microgravity improves proliferation of human iPSC-derived cardiomyocytes
Abstract
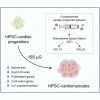
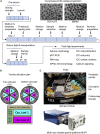
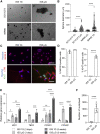
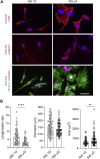
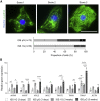
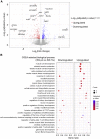
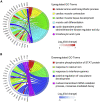
In microgravity, cells undergo profound changes in their properties. However, how human cardiac progenitors respond to space microgravity is unknown. In this study, we evaluated the effect of space microgravity on differentiation of human induced pluripotent stem cell (hiPSC)-derived cardiac progenitors compared with 1G cultures on the International Space Station (ISS). Cryopreserved 3D cardiac progenitors were cultured for 3 weeks on the ISS. Compared with 1G cultures, the microgravity cultures had 3-fold larger sphere sizes, 20-fold higher counts of nuclei, and increased expression of proliferation markers. Highly enriched cardiomyocytes generated in space microgravity showed improved Ca2+ handling and increased expression of contraction-associated genes. Short-term exposure (3 days) of cardiac progenitors to space microgravity upregulated genes involved in cell proliferation, survival, cardiac differentiation, and contraction, consistent with improved microgravity cultures at the late stage. These results indicate that space microgravity increased proliferation of hiPSC-cardiomyocytes, which had appropriate structure and function.
Keywords: calcium handling; cardiac progenitors; cardiomyocytes; differentiation; function; gene experssison; human induced pluripotent stem cells; microgravity; proliferation; spaceflight.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest J.F. and G.B. were employees of Techshot. All other authors declare no competing interests.
Figures

Similar articles
-
Space microgravity increases expression of genes associated with proliferation and differentiation in human cardiac spheres.NPJ Microgravity. 2023 Dec 9;9(1):88. doi: 10.1038/s41526-023-00336-6. NPJ Microgravity. 2023. PMID: 38071377 Free PMC article.
-
Effects of Spaceflight on Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Structure and Function.Stem Cell Reports. 2019 Dec 10;13(6):960-969. doi: 10.1016/j.stemcr.2019.10.006. Epub 2019 Nov 7. Stem Cell Reports. 2019. PMID: 31708475 Free PMC article.
-
Simulated microgravity improves maturation of cardiomyocytes derived from human induced pluripotent stem cells.Sci Rep. 2024 Jan 26;14(1):2243. doi: 10.1038/s41598-024-52453-1. Sci Rep. 2024. PMID: 38278855 Free PMC article.
-
Spaceflight Promoted Myocardial Differentiation of Induced Pluripotent Stem Cells: Results from Tianzhou-1 Space Mission.Stem Cells Dev. 2019 Mar 15;28(6):357-360. doi: 10.1089/scd.2018.0240. Epub 2019 Feb 25. Stem Cells Dev. 2019. PMID: 30654722 Review.
-
Behavior of Stem-Like Cells, Precursors for Tissue Regeneration in Urodela, Under Conditions of Microgravity.Stem Cells Dev. 2019 Apr 1;28(7):423-437. doi: 10.1089/scd.2018.0220. Epub 2019 Feb 28. Stem Cells Dev. 2019. PMID: 30696352 Review.
Cited by
-
Safeguarding Earth's biodiversity by creating a lunar biorepository.Bioscience. 2024 Jul 31;74(8):561-566. doi: 10.1093/biosci/biae058. eCollection 2024 Aug. Bioscience. 2024. PMID: 39229623 Free PMC article.
-
Omics Studies of Tumor Cells under Microgravity Conditions.Int J Mol Sci. 2024 Jan 11;25(2):926. doi: 10.3390/ijms25020926. Int J Mol Sci. 2024. PMID: 38255998 Free PMC article. Review.
-
Discoveries from human stem cell research in space that are relevant to advancing cellular therapies on Earth.NPJ Microgravity. 2024 Aug 21;10(1):88. doi: 10.1038/s41526-024-00425-0. NPJ Microgravity. 2024. PMID: 39168992 Free PMC article. Review.
-
3D cell culture model: From ground experiment to microgravity study.Front Bioeng Biotechnol. 2023 Mar 24;11:1136583. doi: 10.3389/fbioe.2023.1136583. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37034251 Free PMC article. Review.
-
Cardiovascular adaptations and pathological changes induced by spaceflight: from cellular mechanisms to organ-level impacts.Mil Med Res. 2024 Sep 27;11(1):68. doi: 10.1186/s40779-024-00570-3. Mil Med Res. 2024. PMID: 39334239 Free PMC article. Review.
References
-
- Baio J., Martinez A.F., Silva I., Hoehn C.V., Countryman S., Bailey L., Hasaniya N., Pecaut M.J., Kearns-Jonker M. Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. NPJ Microgravity. 2018;4:13. doi: 10.1038/s41526-018-0048-x. - DOI - PMC - PubMed
-
- Baljinnyam E., Venkatesh S., Gordan R., Mareedu S., Zhang J., Xie L.H., Azzam E.I., Suzuki C.K., Fraidenraich D. Effect of densely ionizing radiation on cardiomyocyte differentiation from human-induced pluripotent stem cells. Physiol. Rep. 2017;5:e13308. doi: 10.14814/phy2.13308. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

