Sensing of SARS-CoV-2 by pDCs and their subsequent production of IFN-I contribute to macrophage-induced cytokine storm during COVID-19
- PMID: 36083891
- PMCID: PMC9853436
- DOI: 10.1126/sciimmunol.add4906
Sensing of SARS-CoV-2 by pDCs and their subsequent production of IFN-I contribute to macrophage-induced cytokine storm during COVID-19
Abstract
Lung-infiltrating macrophages create a marked inflammatory milieu in a subset of patients with COVID-19 by producing a cytokine storm, which correlates with increased lethality. However, these macrophages are largely not infected by SARS-CoV-2, so the mechanism underlying their activation in the lung is unclear. Type I interferons (IFN-I) contribute to protecting the host against SARS-CoV-2 but may also have some deleterious effect, and the source of IFN-I in the lungs of infected patients is not well defined. Plasmacytoid dendritic cells (pDCs), a key cell type involved in antiviral responses, can produce IFN-I in response to SARS-CoV-2. We observed the infiltration of pDCs in the lungs of SARS-CoV-2-infected patients, which correlated with strong IFN-I signaling in lung macrophages. In patients with severe COVID-19, lung macrophages expressed a robust inflammatory signature, which correlated with persistent IFN-I signaling at the single-cell level. Hence, we observed the uncoupling in the kinetics of the infiltration of pDCs in the lungs and the associated IFN-I signature, with the cytokine storm in macrophages. We observed that pDCs were the dominant IFN-α-producing cells in response to the virus in the blood, whereas macrophages produced IFN-α only when in physical contact with infected epithelial cells. We also showed that IFN-α produced by pDCs, after the sensing of SARS-CoV-2 by TLR7, mediated changes in macrophages at both transcriptional and epigenetic levels, which favored their hyperactivation by environmental stimuli. Together, these data indicate that the priming of macrophages can result from the response by pDCs to SARS-CoV-2, leading to macrophage activation in patients with severe COVID-19.
Conflict of interest statement
Figures

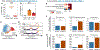
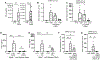

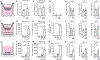

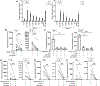

Similar articles
-
Macrophage phagocytosis of SARS-CoV-2-infected cells mediates potent plasmacytoid dendritic cell activation.Cell Mol Immunol. 2023 Jul;20(7):835-849. doi: 10.1038/s41423-023-01039-4. Epub 2023 May 30. Cell Mol Immunol. 2023. PMID: 37253946 Free PMC article.
-
Plasmacytoid dendritic cells produce type I interferon and reduce viral replication in airway epithelial cells after SARS-CoV-2 infection.bioRxiv [Preprint]. 2021 May 13:2021.05.12.443948. doi: 10.1101/2021.05.12.443948. bioRxiv. 2021. PMID: 34013278 Free PMC article. Preprint.
-
High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus.J Virol. 2015 Apr;89(7):3859-69. doi: 10.1128/JVI.03607-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609809 Free PMC article.
-
Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection.Eur J Immunol. 2021 May;51(5):1071-1075. doi: 10.1002/eji.202149173. Epub 2021 Mar 22. Eur J Immunol. 2021. PMID: 33675065 Free PMC article. Review.
-
Plasmacytoid dendritic cells during COVID-19: Ally or adversary?Cell Rep. 2022 Jul 26;40(4):111148. doi: 10.1016/j.celrep.2022.111148. Epub 2022 Jul 14. Cell Rep. 2022. PMID: 35858624 Free PMC article. Review.
Cited by
-
Inflammation durably imprints memory CD4+ T cells.bioRxiv [Preprint]. 2023 May 22:2022.11.15.516351. doi: 10.1101/2022.11.15.516351. bioRxiv. 2023. Update in: Sci Immunol. 2024 Jun 21;9(96):eadj8526. doi: 10.1126/sciimmunol.adj8526 PMID: 36415470 Free PMC article. Updated. Preprint.
-
Interferons and epigenetic mechanisms in training, priming and tolerance of monocytes and hematopoietic progenitors.Immunol Rev. 2024 May;323(1):257-275. doi: 10.1111/imr.13330. Epub 2024 Apr 3. Immunol Rev. 2024. PMID: 38567833 Free PMC article. Review.
-
Immune-profiling of SARS-CoV-2 viremic patients reveals dysregulated innate immune responses.Front Immunol. 2022 Nov 9;13:984553. doi: 10.3389/fimmu.2022.984553. eCollection 2022. Front Immunol. 2022. PMID: 36439166 Free PMC article.
-
Differential platelet activation through an interaction with spike proteins of different SARS-CoV-2 variants.J Thromb Thrombolysis. 2023 Nov;56(4):538-547. doi: 10.1007/s11239-023-02891-x. Epub 2023 Sep 22. J Thromb Thrombolysis. 2023. PMID: 37736784
-
A Comprehensive Analysis of NRP1 in Malignancies Provide Therapeutic Implication for Treating Cancer Patients Infected with SARS-CoV-2.Biochem Genet. 2024 Aug;62(4):2399-2417. doi: 10.1007/s10528-023-10518-2. Epub 2023 Nov 8. Biochem Genet. 2024. PMID: 37938510
References
-
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z, Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med 26, 842–844 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

