Alpha-Mangostin-Loaded Transferrin-Conjugated Lipid-Polymer Hybrid Nanoparticles: Development and Characterization for Tumor-Targeted Delivery
- PMID: 36081606
- PMCID: PMC9448606
- DOI: 10.1155/2022/9217268
Alpha-Mangostin-Loaded Transferrin-Conjugated Lipid-Polymer Hybrid Nanoparticles: Development and Characterization for Tumor-Targeted Delivery
Abstract
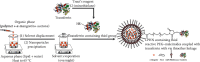
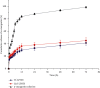
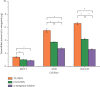
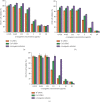
Alpha-mangostin, a natural xanthone mainly extracted from the pericarp of Garcinia mangostana, has been shown to have promising anticancer properties in many types of cancer. However, the therapeutic potential of α-mangostin has been limited so far due to its poor aqueous solubility and low oral bioavailability, which limited its biopharmaceutical applications. Furthermore, α-mangostin failed to specifically reach tumors at a therapeutic concentration due and rapid elimination in vivo. We hypothesized that this drawback could be overcome by loading the drug within a delivery system conjugated to transferrin (Tf), whose receptors are overexpressed on many cancer cells and would enhance the specific delivery of α-mangostin to cancer cells, thereby enhancing its therapeutic efficacy. The objectives of this study were therefore to prepare and characterize transferrin-conjugated lipid-polymer hybrid nanoparticles (LPHN) entrapping α-mangostin, as well as to evaluate their therapeutic efficacy in vitro. We successfully prepared α-mangostin loaded LPHN using a one-step nanoprecipitation method with high drug entrapment efficiency. The conjugation of Tf to the LPHN was achieved by using the thiol-maleimide "click" reaction, leading to an increase in the particle hydrodynamic size of Tf-LPHN compared to that of unconjugated (control) LPHN (Ctrl-LPHN). Both Tf-LPHN and Ctrl-LPHN were bearing negative surface charges. Tf-LPHN and Ctrl-LPHN exhibited a sustained release of α-mangostin at pH 7.4, following an initial burst release, unlike rapid release of drug solution. The entrapment of α-mangostin in the LPHN led to an increase in α-mangostin uptake by cancer cells, and thus improved its antiproliferative activity compared to that observed with the drug solution. In conclusion, α-mangostin entrapped in the Tf-LPHN is therefore a highly promising therapeutic system that should be further optimized as therapeutic tools for cancer treatment.
Copyright © 2022 Intouch Sakpakdeejaroen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Nanoformulations of α-Mangostin for Cancer Drug Delivery System.Pharmaceutics. 2021 Nov 24;13(12):1993. doi: 10.3390/pharmaceutics13121993. Pharmaceutics. 2021. PMID: 34959275 Free PMC article. Review.
-
Nanoparticle Drug Delivery Systems for α-Mangostin.Nanotechnol Sci Appl. 2020 Apr 1;13:23-36. doi: 10.2147/NSA.S243017. eCollection 2020. Nanotechnol Sci Appl. 2020. PMID: 32280205 Free PMC article. Review.
-
Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy.Colloids Surf B Biointerfaces. 2019 Sep 1;181:705-713. doi: 10.1016/j.colsurfb.2019.06.011. Epub 2019 Jun 6. Colloids Surf B Biointerfaces. 2019. PMID: 31228853
-
Transferrin-modified liposome promotes α-mangostin to penetrate the blood-brain barrier.Nanomedicine. 2016 Feb;12(2):421-30. doi: 10.1016/j.nano.2015.10.021. Epub 2015 Dec 19. Nanomedicine. 2016. PMID: 26711963
-
Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid-Polymer Hybrid Nanoparticles.Int J Nanomedicine. 2021 Apr 6;16:2615-2631. doi: 10.2147/IJN.S293480. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33854311 Free PMC article.
Cited by
-
Preparation and evaluation of a niosomal delivery system containing G. mangostana extract and study of its anti-Acanthamoeba activity.Nanoscale Adv. 2024 Jan 9;6(5):1467-1479. doi: 10.1039/d3na01016c. eCollection 2024 Feb 27. Nanoscale Adv. 2024. PMID: 38419876 Free PMC article.
-
Cytotoxicity Enhancement of α-Mangostin with Folate-Conjugated Chitosan Nanoparticles in MCF-7 Breast Cancer Cells.Molecules. 2023 Nov 14;28(22):7585. doi: 10.3390/molecules28227585. Molecules. 2023. PMID: 38005306 Free PMC article.
-
Surface Functionalised Parenteral Nanoemulsions for Active and Homotypic Targeting to Melanoma.Pharmaceutics. 2023 Apr 28;15(5):1358. doi: 10.3390/pharmaceutics15051358. Pharmaceutics. 2023. PMID: 37242600 Free PMC article.
References
-
- Ibrahim M. Y., Hashim N. M., Mariod A. A., et al. α-Mangostin from Garcinia mangostana Linn: an updated review of its pharmacological properties. Arabian Journal of Chemistry . 2016;9(3):317–329. doi: 10.1016/j.arabjc.2014.02.011. - DOI
-
- Muchtaridi M., Wijaya C. A. Anticancer potential of α-mangostin. Asian Journal of Pharmaceutical and Clinical Research . 2017;10(12):p. 440. doi: 10.22159/ajpcr.2017.v10i12.20812. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

