Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice
- PMID: 36080365
- PMCID: PMC9458100
- DOI: 10.3390/molecules27175598
Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice
Abstract
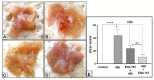
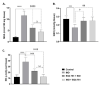
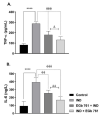
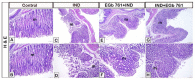
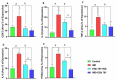
The main bioactive constituents in the standardized Ginkgo biloba leaf extract (EGb 761) are the terpene lactones and flavonoid glycosides. EGb 761's antioxidant and anti-inflammatory properties have previously been demonstrated. Indomethacin-induced gastric ulcers have a multifactorial etiology and represent a major restriction to its therapeutic utility. The underlying ulcerogenic process involves oxidative and inflammatory biomolecular insults. This study was performed to explore the curative and preventative benefits of EGb 761 in experimentally-induced ulcers. To develop gastric ulcers in mice, indomethacin (40 mg/kg) was administered orally. EGb 761 (200 mg/kg) was given by gavage for 7 days before (preventative) and after (therapeutic) indomethacin administration. The histological alterations and macroscopic mucosal lesions were assessed. In gastric tissue homogenates, malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide (NO), and inflammatory cytokines were measured. The expressions of cyclooxygenase-2 (COX-2), cytokines, and proliferating cell nuclear antigen (PCNA) in the stomach mucosa were also investigated. The ulcer index, histological alterations, gastric oxidants, and inflammatory biomarkers were all significantly increased by indomethacin. In stomach specimens, it increased COX-2 and PCNA expression. EGb 761 treatments, both prophylactic and therapeutic, resulted in significant reductions in ulcer lesions, nitrosative and oxidative damage, and inflammatory markers, along with the lowering of COX-2 and PCNA expressions. Furthermore, in the fight against stomach ulcers, EGb 761 treatment was found to be more efficient than prevention.
Keywords: EGb 761; indomethacin; mice; preventative; therapeutic; ulcer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Apium extract alleviates indomethacin-induced gastric ulcers in rats via modulating the VEGF and IK-κB/NF-κB p65 signaling pathway: insights from in silico and in vivo investigations.BMC Complement Med Ther. 2024 Feb 14;24(1):88. doi: 10.1186/s12906-023-04333-w. BMC Complement Med Ther. 2024. PMID: 38355510 Free PMC article.
-
Syzygium samarangense leaf extract mitigates indomethacin-induced gastropathy via the NF-κB signaling pathway in rats.Biomed Pharmacother. 2021 Jul;139:111675. doi: 10.1016/j.biopha.2021.111675. Epub 2021 May 6. Biomed Pharmacother. 2021. PMID: 33965725
-
Gastroprotective Effects of Spirulina platensis, Golden Kiwifruit Flesh, and Golden Kiwifruit Peel Extracts Individually or in Combination against Indomethacin-Induced Gastric Ulcer in Rats.Nutrients. 2021 Oct 3;13(10):3499. doi: 10.3390/nu13103499. Nutrients. 2021. PMID: 34684501 Free PMC article.
-
EGb 761: ginkgo biloba extract, Ginkor.Drugs R D. 2003;4(3):188-93. doi: 10.2165/00126839-200304030-00009. Drugs R D. 2003. PMID: 12757407 Review.
-
Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer's disease.Pharmacopsychiatry. 2003 Jun;36 Suppl 1:S8-14. doi: 10.1055/s-2003-40454. Pharmacopsychiatry. 2003. PMID: 13130383 Review.
Cited by
-
Pumpkin seed oil: unveiling its potential in controlling inflammation and pathogenicity during experimental trichinellosis.BMC Vet Res. 2024 Sep 20;20(1):419. doi: 10.1186/s12917-024-04241-2. BMC Vet Res. 2024. PMID: 39304848 Free PMC article.
-
Microstructural architecture of the bony scutes, spine, and rays of the bony fins in the common pleco (Hypostomus plecostomus).Int J Vet Sci Med. 2024 Sep 4;12(1):101-124. doi: 10.1080/23144599.2024.2374201. eCollection 2024. Int J Vet Sci Med. 2024. PMID: 39239634 Free PMC article.
-
Modulation of the intestinal mucosal and cell-mediated response against natural helminth infection in the African catfish Clarias gariepinus.BMC Vet Res. 2024 Jul 27;20(1):335. doi: 10.1186/s12917-024-04153-1. BMC Vet Res. 2024. PMID: 39068442 Free PMC article.
-
Immunohistochemical properties of embryonic telocytes in a myogenic microenvironment.Sci Rep. 2024 May 27;14(1):12034. doi: 10.1038/s41598-024-62103-1. Sci Rep. 2024. PMID: 38802438 Free PMC article.
-
Tamarix aphylla derived metabolites ameliorate indomethacin-induced gastric ulcers in rats by modulating the MAPK signaling pathway, alleviating oxidative stress and inflammation: In vivo study supported by pharmacological network analysis.PLoS One. 2024 May 10;19(5):e0302015. doi: 10.1371/journal.pone.0302015. eCollection 2024. PLoS One. 2024. PMID: 38728332 Free PMC article.
References
-
- Tomić M., Micov A., Pecikoza U., Stepanović-Petrović R. Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs. Challenges Potential Benefits; Academic Press; Cambridge, MA, USA: 2017. Clinical Uses of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Potential Benefits of NSAIDs Modified-Release Preparations; pp. 1–29. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

