Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis
- PMID: 36077437
- PMCID: PMC9456451
- DOI: 10.3390/ijms231710034
Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis
Abstract
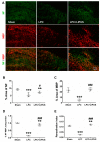
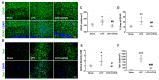
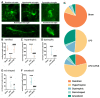
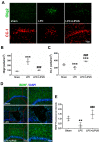
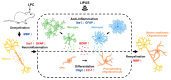
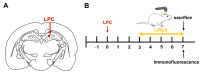
Microglia, astrocytes, and oligodendrocyte progenitor cells (OPCs) may serve as targets for remyelination-enhancing therapy. Low-intensity pulsed ultrasound (LIPUS) has been demonstrated to ameliorate myelin loss and inhibit neuroinflammation in animal models of brain disorders; however, the underlying mechanisms through which LIPUS stimulates remyelination and glial activation are not well-understood. This study explored the impacts of LIPUS on remyelination and resident cells following lysolecithin (LPC)-induced local demyelination in the hippocampus. Demyelination was induced by the micro-injection of 1.5 μL of 1% LPC into the rat hippocampus, and the treatment groups received daily LIPUS stimulation for 5 days. The therapeutic effects of LIPUS on LPC-induced demyelination were assessed through immunohistochemistry staining. The staining was performed to evaluate remyelination and Iba-1 staining as a microglia marker. Our data revealed that LIPUS significantly increased myelin basic protein (MBP) expression. Moreover, the IHC results showed that LIPUS significantly inhibited glial cell activation, enhanced mature oligodendrocyte density, and promoted brain-derived neurotrophic factor (BDNF) expression at the lesion site. In addition, a heterologous population of microglia with various morphologies can be found in the demyelination lesion after LIPUS treatment. These data show that LIPUS stimulation may serve as a potential treatment for accelerating remyelination through the attenuation of glial activation and the enhancement of mature oligodendrocyte density and BDNF production.
Keywords: demyelination; microglia; multiple sclerosis; oligodendrocyte; remyelination; ultrasound.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Dynamic response of microglia/macrophage polarization following demyelination in mice.J Neuroinflammation. 2019 Oct 17;16(1):188. doi: 10.1186/s12974-019-1586-1. J Neuroinflammation. 2019. PMID: 31623610 Free PMC article.
-
Magnetization transfer ratio for assessing remyelination after transcranial ultrasound stimulation in the lysolecithin rat model of multiple sclerosis.Cereb Cortex. 2023 Feb 7;33(4):1403-1411. doi: 10.1093/cercor/bhac144. Cereb Cortex. 2023. PMID: 35368059
-
Loss of Tuberous Sclerosis Complex1 in Adult Oligodendrocyte Progenitor Cells Enhances Axon Remyelination and Increases Myelin Thickness after a Focal Demyelination.J Neurosci. 2017 Aug 2;37(31):7534-7546. doi: 10.1523/JNEUROSCI.3454-16.2017. Epub 2017 Jul 10. J Neurosci. 2017. PMID: 28694334 Free PMC article.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis.Glia. 2022 Jul;70(7):1215-1250. doi: 10.1002/glia.24148. Epub 2022 Feb 2. Glia. 2022. PMID: 35107839 Free PMC article. Review.
Cited by
-
Neuronal activity and remyelination: new insights into the molecular mechanisms and therapeutic advancements.Front Cell Dev Biol. 2023 Jul 26;11:1221890. doi: 10.3389/fcell.2023.1221890. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37564376 Free PMC article. Review.
-
Knowledge mapping and global trends in the field of low-intensity pulsed ultrasound and endocrine and metabolic diseases: a bibliometric and visual analysis from 2012 to 2022.Front Endocrinol (Lausanne). 2023 Sep 5;14:1237864. doi: 10.3389/fendo.2023.1237864. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37732128 Free PMC article.
-
Neuroprotection by Abdominal Ultrasound in Lipopolysaccharide-Induced Systemic Inflammation.Int J Mol Sci. 2023 May 26;24(11):9329. doi: 10.3390/ijms24119329. Int J Mol Sci. 2023. PMID: 37298275 Free PMC article.
References
-
- Ciccarelli O., Barkhof F., Bodini B., De Stefano N., Golay X., Nicolay K., Pelletier D., Pouwels P.J., Smith S.A., Wheeler-Kingshott C.A., et al. Pathogenesis of multiple sclerosis: Insights from molecular and metabolic imaging. Lancet Neurol. 2014;13:807–822. doi: 10.1016/S1474-4422(14)70101-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

