Novel Triterpenic Acid-Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents
- PMID: 36077389
- PMCID: PMC9456456
- DOI: 10.3390/ijms23179992
Novel Triterpenic Acid-Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents
Abstract
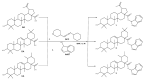
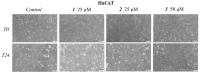
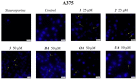
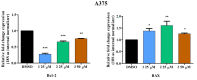
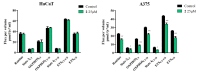
Pentacyclic triterpenes, such as betulinic, ursolic, and oleanolic acids are efficient and selective anticancer agents whose underlying mechanisms of action have been widely investigated. The introduction of N-bearing heterocycles (e.g., triazoles) into the structures of natural compounds (particularly pentacyclic triterpenes) has yielded semisynthetic derivatives with increased antiproliferative potential as opposed to unmodified starting compounds. In this work, we report the synthesis and biological assessment of benzotriazole esters of betulinic acid (BA), oleanolic acid (OA), and ursolic acid (UA) (compounds 1-3). The esters were obtained in moderate yields (28-42%). All three compounds showed dose-dependent reductions in cell viability against A375 melanoma cells and no cytotoxic effects against healthy human keratinocytes. The morphology analysis of treated cells showed characteristic apoptotic changes consisting of nuclear shrinkage, condensation, fragmentation, and cellular membrane disruption. rtPCR analysis reinforced the proapoptotic evidence, showing a reduction in anti-apoptotic Bcl-2 expression and upregulation of the pro-apoptotic Bax. High-resolution respirometry studies showed that all three compounds were able to significantly inhibit mitochondrial function. Molecular docking showed that compounds 1-3 showed an increase in binding affinity against Bcl-2 as opposed to BA, OA, and UA and similar binding patterns compared to known Bcl-2 inhibitors.
Keywords: 1-hydroxybenzotriazole esters; apoptosis; cytotoxicity; melanoma; molecular docking; rtPCR; triterpenic acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



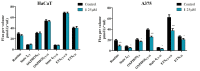
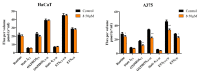

Similar articles
-
The Antimelanoma Biological Assessment of Triterpenic Acid Functionalized Gold Nanoparticles.Molecules. 2023 Jan 3;28(1):421. doi: 10.3390/molecules28010421. Molecules. 2023. PMID: 36615613 Free PMC article.
-
Betulinic, oleanolic and ursolic acids inhibit the enzymatic and biological effects induced by a P-I snake venom metalloproteinase.Chem Biol Interact. 2018 Jan 5;279:219-226. doi: 10.1016/j.cbi.2017.12.001. Epub 2017 Dec 5. Chem Biol Interact. 2018. PMID: 29203373
-
Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion?J Agric Food Chem. 2016 Apr 20;64(15):2991-3008. doi: 10.1021/acs.jafc.5b06021. Epub 2016 Apr 8. J Agric Food Chem. 2016. PMID: 27012451 Review.
-
Selective in vitro anti-melanoma activity of ursolic and oleanolic acids.Toxicol Mech Methods. 2018 Feb;28(2):148-156. doi: 10.1080/15376516.2017.1373881. Epub 2017 Sep 29. Toxicol Mech Methods. 2018. PMID: 28868958
-
Semisynthetic Derivatives of Pentacyclic Triterpenes Bearing Heterocyclic Moieties with Therapeutic Potential.Molecules. 2022 Oct 3;27(19):6552. doi: 10.3390/molecules27196552. Molecules. 2022. PMID: 36235089 Free PMC article. Review.
Cited by
-
The C30-Modulation of Betulinic Acid Using 1,2,4-Triazole: A Promising Strategy for Increasing Its Antimelanoma Cytotoxic Potential.Molecules. 2022 Nov 12;27(22):7807. doi: 10.3390/molecules27227807. Molecules. 2022. PMID: 36431906 Free PMC article.
-
Chemical and cytotoxicity profiles of 11 pink pepper (Schinus spp.) samples via non-targeted hyphenated high-performance thin-layer chromatography.Metabolomics. 2023 May 2;19(5):48. doi: 10.1007/s11306-023-02008-8. Metabolomics. 2023. PMID: 37130976 Free PMC article.
-
Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis.Molecules. 2024 Jun 28;29(13):3091. doi: 10.3390/molecules29133091. Molecules. 2024. PMID: 38999041 Free PMC article. Review.
-
Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications.Molecules. 2023 Nov 24;28(23):7763. doi: 10.3390/molecules28237763. Molecules. 2023. PMID: 38067491 Free PMC article. Review.
-
The Antimelanoma Biological Assessment of Triterpenic Acid Functionalized Gold Nanoparticles.Molecules. 2023 Jan 3;28(1):421. doi: 10.3390/molecules28010421. Molecules. 2023. PMID: 36615613 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

