Lactobacillus rhamnosus GR-1 attenuates foodborne Bacillus cereus-induced NLRP3 inflammasome activity in bovine mammary epithelial cells by protecting intercellular tight junctions
- PMID: 36076276
- PMCID: PMC9461272
- DOI: 10.1186/s40104-022-00752-w
Lactobacillus rhamnosus GR-1 attenuates foodborne Bacillus cereus-induced NLRP3 inflammasome activity in bovine mammary epithelial cells by protecting intercellular tight junctions
Abstract
Background: Bacillus cereus is an important pathogen that causes human food poisoning, specifically diarrhea and vomiting. B. cereus can also induce mastitis in dairy cows and has a strong survival ability in milk, as it cannot be inactivated by high-temperature short-time pasteurization. Therefore, B. cereus can enter the market through pasteurized milk and other dairy products, imposing enormous hidden dangers on food safety and human health.
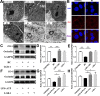
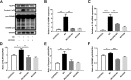
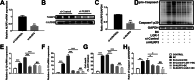
Results: In this study, B. cereus 2101 (BC) was isolated from milk samples of cows with mastitis. BC grew rapidly with strong hemolysis, making it difficult to prevent mastitis and ensure food security. MAC-T cells were treated with BC and/or Lactobacillus rhamnosus GR-1 (LGR-1). Pretreatment with LGR-1 protected the integrity of tight junctions and the expression of zonula occludens-1 (ZO-1) and occludin destroyed by BC. Furthermore, LGR-1 pretreatment reduced the expression of NOD-like receptor family member pyrin domain-containing protein 3 (NLRP3), caspase recruitment and activation domain (ASC), Caspase-1 p20, gasdermin D (GSDMD) p30, inflammatory factors (interleukin (IL)-1β and IL-18), and cell death induced by BC. Moreover, LGR-1 pretreatment reduced NLRP3 inflammasome activity and increased expressions of ZO-1 and occludin induced by lipopolysaccharides (LPS) + ATP stimulation. MAC-T cells were transfected with NLRP3 siRNA or MCC950 and/or treated with BC and/or LGR-1. NLRP3-siRNA transfection and MCC950 attenuated BC-induced NLRP3 inflammasome activity. Expression of inflammatory cytokines and cell death suggested that the inflammatory pathway might play an important role in the induction of the NLRP3 inflammasome by BC and the protection of LGR-1.
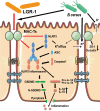
Conclusions: These results suggest that LGR-1 might be a probiotic alternative to antibiotics and could be administered to prevent mastitis in dairy cows, thus ensuring food security.
Keywords: Bacillus cereus; Intercellular tight junctions; Lactobacillus rhamnosus GR-1; NLRP3 inflammasome.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Lactobacillus rhamnosus GR-1 Prevents Escherichia coli-Induced Apoptosis Through PINK1/Parkin-Mediated Mitophagy in Bovine Mastitis.Front Immunol. 2021 Sep 14;12:715098. doi: 10.3389/fimmu.2021.715098. eCollection 2021. Front Immunol. 2021. PMID: 34594329 Free PMC article.
-
Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation.Appl Environ Microbiol. 2015 Dec 11;82(4):1173-1182. doi: 10.1128/AEM.03044-15. Print 2016 Feb 15. Appl Environ Microbiol. 2015. PMID: 26655757 Free PMC article.
-
Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Activation of NLRP3 and NLRC4 Inflammasomes With Differential Requirement for ASC.Front Microbiol. 2018 Jul 24;9:1661. doi: 10.3389/fmicb.2018.01661. eCollection 2018. Front Microbiol. 2018. PMID: 30087667 Free PMC article.
-
Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis.Microorganisms. 2022 Jan 10;10(1):137. doi: 10.3390/microorganisms10010137. Microorganisms. 2022. PMID: 35056585 Free PMC article.
-
Non-canonical Inflammasome-Mediated IL-1β Production by Primary Endometrial Epithelial and Stromal Fibroblast Cells Is NLRP3 and Caspase-4 Dependent.Front Immunol. 2019 Feb 5;10:102. doi: 10.3389/fimmu.2019.00102. eCollection 2019. Front Immunol. 2019. PMID: 30804935 Free PMC article.
Cited by
-
Bioactive Compounds and Probiotics Mitigate Mastitis by Targeting NF-κB Signaling Pathway.Biomolecules. 2024 Aug 15;14(8):1011. doi: 10.3390/biom14081011. Biomolecules. 2024. PMID: 39199398 Free PMC article. Review.
-
Breaking the Gingival Barrier in Periodontitis.Int J Mol Sci. 2023 Feb 25;24(5):4544. doi: 10.3390/ijms24054544. Int J Mol Sci. 2023. PMID: 36901974 Free PMC article. Review.
-
Revealing the Mechanism of NLRP3 Inflammatory Pathway Activation through K+ Efflux Induced by PLO via Signal Point Mutations.Int J Mol Sci. 2024 Jun 18;25(12):6703. doi: 10.3390/ijms25126703. Int J Mol Sci. 2024. PMID: 38928408 Free PMC article.
-
Angelica acutiloba Kitagawa flower induces A549 cell pyroptosis via the NF-κB/NLRP3 pathway for anti-lung cancer effects.Cell Div. 2023 Nov 1;18(1):19. doi: 10.1186/s13008-023-00102-w. Cell Div. 2023. PMID: 37907950 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

