Early impairment of cortical circuit plasticity and connectivity in the 5XFAD Alzheimer's disease mouse model
- PMID: 36075886
- PMCID: PMC9458752
- DOI: 10.1038/s41398-022-02132-4
Early impairment of cortical circuit plasticity and connectivity in the 5XFAD Alzheimer's disease mouse model
Abstract
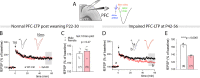
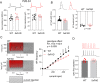
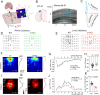
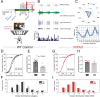
Genetic risk factors for neurodegenerative disorders, such as Alzheimer's disease (AD), are expressed throughout the life span. How these risk factors affect early brain development and function remain largely unclear. Analysis of animal models with high constructive validity for AD, such as the 5xFAD mouse model, may provide insights on potential early neurodevelopmental effects that impinge on adult brain function and age-dependent degeneration. The 5XFAD mouse model over-expresses human amyloid precursor protein (APP) and presenilin 1 (PS1) harboring five familial AD mutations. It is unclear how the expression of these mutant proteins affects early developing brain circuits. We found that the prefrontal cortex (PFC) layer 5 (L5) neurons in 5XFAD mice exhibit transgenic APP overloading at an early post-weaning age. Impaired synaptic plasticity (long-term potentiation, LTP) was seen at 6-8 weeks age in L5 PFC circuit, which was correlated with increased intracellular APP. APP overloading was also seen in L5 pyramidal neurons in the primary visual cortex (V1) during the critical period of plasticity (4-5 weeks age). Whole-cell patch clamp recording in V1 brain slices revealed reduced intrinsic excitability of L5 neurons in 5XFAD mice, along with decreased spontaneous miniature excitatory and inhibitory inputs. Functional circuit mapping using laser scanning photostimulation (LSPS) combined with glutamate uncaging uncovered reduced excitatory synaptic connectivity onto L5 neurons in V1, and a more pronounced reduction in inhibitory connectivity, indicative of altered excitation and inhibition during VC critical period. Lastly, in vivo single-unit recording in V1 confirmed that monocular visual deprivation-induced ocular dominance plasticity during critical period was impaired in 5XFAD mice. Our study reveals plasticity deficits across multiple cortical regions and indicates altered early cortical circuit developmental trajectory as a result of mutant APP/PS1 over-expression.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Disrupted Maturation of Prefrontal Layer 5 Neuronal Circuits in an Alzheimer's Mouse Model of Amyloid Deposition.Neurosci Bull. 2023 Jun;39(6):881-892. doi: 10.1007/s12264-022-00951-5. Epub 2022 Sep 24. Neurosci Bull. 2023. PMID: 36152121 Free PMC article.
-
Depletion of microglia in developing cortical circuits reveals its critical role in glutamatergic synapse development, functional connectivity, and critical period plasticity.J Neurosci Res. 2020 Oct;98(10):1968-1986. doi: 10.1002/jnr.24641. Epub 2020 Jun 28. J Neurosci Res. 2020. PMID: 32594561
-
Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice.J Neurosci. 2002 May 1;22(9):3445-53. doi: 10.1523/JNEUROSCI.22-09-03445.2002. J Neurosci. 2002. PMID: 11978821 Free PMC article.
-
Presenilins and APP in neuritic and synaptic plasticity: implications for the pathogenesis of Alzheimer's disease.Neuromolecular Med. 2002;2(2):167-96. doi: 10.1385/NMM:2:2:167. Neuromolecular Med. 2002. PMID: 12428810 Review.
-
Hippocampal Deficits in Amyloid-β-Related Rodent Models of Alzheimer's Disease.Front Neurosci. 2020 Apr 7;14:266. doi: 10.3389/fnins.2020.00266. eCollection 2020. Front Neurosci. 2020. PMID: 32317913 Free PMC article. Review.
Cited by
-
Firing Alterations of Neurons in Alzheimer's Disease: Are They Merely a Consequence of Pathogenesis or a Pivotal Component of Disease Progression?Cells. 2024 Feb 29;13(5):434. doi: 10.3390/cells13050434. Cells. 2024. PMID: 38474398 Free PMC article. Review.
-
Immunotherapy with Cleavage-Specific 12A12mAb Reduces the Tau Cleavage in Visual Cortex and Improves Visuo-Spatial Recognition Memory in Tg2576 AD Mouse Model.Pharmaceutics. 2023 Feb 3;15(2):509. doi: 10.3390/pharmaceutics15020509. Pharmaceutics. 2023. PMID: 36839831 Free PMC article.
-
Seven Tesla MRI in Alzheimer's disease research: State of the art and future directions: A narrative review.AIMS Neurosci. 2023 Dec 11;10(4):401-422. doi: 10.3934/Neuroscience.2023030. eCollection 2023. AIMS Neurosci. 2023. PMID: 38188012 Free PMC article. Review.
-
Entorhinal cortex vulnerability to human APP expression promotes hyperexcitability and tau pathology.Nat Commun. 2024 Sep 10;15(1):7918. doi: 10.1038/s41467-024-52297-3. Nat Commun. 2024. PMID: 39256379 Free PMC article.
-
Entorhinal cortex vulnerability to human APP expression promotes hyperexcitability and tau pathology.Res Sq [Preprint]. 2023 Nov 6:rs.3.rs-3370607. doi: 10.21203/rs.3.rs-3370607/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Sep 10;15(1):7918. doi: 10.1038/s41467-024-52297-3. PMID: 37987015 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

