Nanomaterials: A powerful tool for tumor immunotherapy
- PMID: 36072591
- PMCID: PMC9441741
- DOI: 10.3389/fimmu.2022.979469
Nanomaterials: A powerful tool for tumor immunotherapy
Abstract
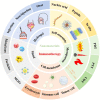
Cancer represents the leading global driver of death and is recognized as a critical obstacle to increasing life expectancy. In recent years, with the development of precision medicine, significant progress has been made in cancer treatment. Among them, various therapies developed with the help of the immune system have succeeded in clinical treatment, recognizing and killing cancer cells by stimulating or enhancing the body's intrinsic immune system. However, low response rates and serious adverse effects, among others, have limited the use of immunotherapy. It also poses problems such as drug resistance and hyper-progression. Fortunately, thanks to the rapid development of nanotechnology, engineered multifunctional nanomaterials and biomaterials have brought breakthroughs in cancer immunotherapy. Unlike conventional cancer immunotherapy, nanomaterials can be rationally designed to trigger specific tumor-killing effects. Simultaneously, improved infiltration of immune cells into metastatic lesions enhances the efficiency of antigen submission and induces a sustained immune reaction. Such a strategy directly reverses the immunological condition of the primary tumor, arrests metastasis and inhibits tumor recurrence through postoperative immunotherapy. This paper discusses several types of nanoscale biomaterials for cancer immunotherapy, and they activate the immune system through material-specific advantages to provide novel therapeutic strategies. In summary, this article will review the latest advances in tumor immunotherapy based on self-assembled, mesoporous, cell membrane modified, metallic, and hydrogel nanomaterials to explore diverse tumor therapies.
Keywords: anti-tumor; immunosuppression; immunotherapy; nanomaterials; tumor microenvironment.
Copyright © 2022 Chen, Yue, Wang, Yang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Nanomaterials: small particles show huge possibilities for cancer immunotherapy.J Nanobiotechnology. 2022 Nov 16;20(1):484. doi: 10.1186/s12951-022-01692-3. J Nanobiotechnology. 2022. PMID: 36384524 Free PMC article. Review.
-
Nanomaterials: leading immunogenic cell death-based cancer therapies.Front Immunol. 2024 Aug 9;15:1447817. doi: 10.3389/fimmu.2024.1447817. eCollection 2024. Front Immunol. 2024. PMID: 39185425 Free PMC article. Review.
-
Nanoscale Metal-Organic Frameworks for Cancer Immunotherapy.Acc Chem Res. 2020 Sep 15;53(9):1739-1748. doi: 10.1021/acs.accounts.0c00313. Epub 2020 Aug 18. Acc Chem Res. 2020. PMID: 32808760 Free PMC article.
-
Stimuli-Responsive Nanomaterials for Tumor Immunotherapy.ACS Biomater Sci Eng. 2024 Sep 9;10(9):5474-5495. doi: 10.1021/acsbiomaterials.4c00388. Epub 2024 Aug 22. ACS Biomater Sci Eng. 2024. PMID: 39171865 Review.
-
Advances of functional nanomaterials for cancer immunotherapeutic applications.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Mar;12(2):e1574. doi: 10.1002/wnan.1574. Epub 2019 Sep 30. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020. PMID: 31566896 Review.
Cited by
-
The role of cGAS in epithelial dysregulation in inflammatory bowel disease and gastrointestinal malignancies.Front Pharmacol. 2024 Jul 10;15:1409683. doi: 10.3389/fphar.2024.1409683. eCollection 2024. Front Pharmacol. 2024. PMID: 39050748 Free PMC article. Review.
-
Carbon Nanomaterials: Emerging Roles in Immuno-Oncology.Int J Mol Sci. 2023 Apr 1;24(7):6600. doi: 10.3390/ijms24076600. Int J Mol Sci. 2023. PMID: 37047572 Free PMC article. Review.
-
Synergistic Photothermal Therapy and Chemotherapy Enabled by Tumor Microenvironment-Responsive Targeted SWCNT Delivery.Int J Mol Sci. 2024 Aug 23;25(17):9177. doi: 10.3390/ijms25179177. Int J Mol Sci. 2024. PMID: 39273127 Free PMC article.
-
Immune checkpoint blockade for locally advanced or recurrent/metastatic cervical cancer: An update on clinical data.Front Oncol. 2022 Dec 22;12:1045481. doi: 10.3389/fonc.2022.1045481. eCollection 2022. Front Oncol. 2022. PMID: 36644634 Free PMC article. Review.
-
PI3K/AKT/mTOR and PD‑1/CTLA‑4/CD28 pathways as key targets of cancer immunotherapy (Review).Oncol Lett. 2024 Sep 26;28(6):567. doi: 10.3892/ol.2024.14700. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39390982 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

