Water-assisted and protein-initiated fast and controlled ring-opening polymerization of proline N-carboxyanhydride
- PMID: 36072505
- PMCID: PMC9438472
- DOI: 10.1093/nsr/nwac033
Water-assisted and protein-initiated fast and controlled ring-opening polymerization of proline N-carboxyanhydride
Abstract
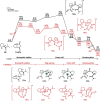
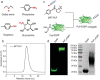
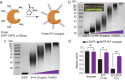
The production of polypeptides via the ring-opening polymerization (ROP) of N-carboxyanhydride (NCA) is usually conducted under stringent anhydrous conditions. The ROP of proline NCA (ProNCA) for the synthesis of poly-L-proline (PLP) is particularly challenging due to the premature product precipitation as polyproline type I helices, leading to slow reactions for up to one week, poor control of the molar mass and laborious workup. Here, we report the unexpected water-assisted controlled ROP of ProNCA, which affords well-defined PLP as polyproline II helices in 2-5 minutes and almost-quantitative yields. Experimental and theoretical studies together suggest the as-yet-unreported role of water in facilitating proton shift, which significantly lowers the energy barrier of the chain propagation. The scope of initiators can be expanded from hydrophobic amines to encompass hydrophilic amines and thiol-bearing nucleophiles, including complex biomacromolecules such as proteins. Protein-mediated ROP of ProNCA conveniently affords various protein-PLP conjugates via a grafting-from approach. PLP modification not only preserves the biological activities of the native proteins, but also enhances their resistance to extreme conditions. Moreover, PLP modification extends the elimination half-life of asparaginase (ASNase) 18-fold and mitigates the immunogenicity of wt ASNase >250-fold (ASNase is a first-line anticancer drug for lymphoma treatment). This work provides a simple solution to a long-standing problem in PLP synthesis, and offers valuable guidance for the development of water-resistant ROP of other proline-like NCAs. The facile access to PLP can greatly boost the application potential of PLP-based functional materials for engineering industry enzymes and therapeutic proteins.
Keywords: N-carboxyanhydride; PPII helix; proline; protein-polymer conjugates; ring-opening polymerization.
© The Author(s) 2022. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures



Similar articles
-
Open-vessel polymerization of N-carboxyanhydride (NCA) for polypeptide synthesis.Nat Protoc. 2024 Oct 8. doi: 10.1038/s41596-024-01062-3. Online ahead of print. Nat Protoc. 2024. PMID: 39379616 Review.
-
Accelerated Polypeptide Synthesis via N-Carboxyanhydride Ring Opening Polymerization in Continuous Flow.Macromol Rapid Commun. 2020 Sep;41(18):e2000071. doi: 10.1002/marc.202000071. Epub 2020 Jul 20. Macromol Rapid Commun. 2020. PMID: 32691465
-
Precision Synthesis of Polysarcosine via Controlled Ring-Opening Polymerization of N-Carboxyanhydride: Fast Kinetics, Ultrahigh Molecular Weight, and Mechanistic Insights.J Am Chem Soc. 2024 Feb 28;146(8):5678-5692. doi: 10.1021/jacs.3c14740. Epub 2024 Feb 15. J Am Chem Soc. 2024. PMID: 38359327
-
Zn(OAc)₂-Catalyzing Ring-Opening Polymerization of N-Carboxyanhydrides for the Synthesis of Well-Defined Polypeptides.Molecules. 2018 Mar 26;23(4):760. doi: 10.3390/molecules23040760. Molecules. 2018. PMID: 29587473 Free PMC article.
-
Polypeptide films via N-carboxyanhydride ring-opening polymerization (NCA-ROP): past, present and future.Chem Commun (Camb). 2014 May 21;50(39):4971-88. doi: 10.1039/c4cc00293h. Epub 2014 Feb 27. Chem Commun (Camb). 2014. PMID: 24577357 Review.
Cited by
-
Clickable Polyprolines from Azido-proline N-Carboxyanhydride.ACS Polym Au. 2023 Jul 16;3(5):383-393. doi: 10.1021/acspolymersau.3c00011. eCollection 2023 Oct 11. ACS Polym Au. 2023. PMID: 37841952 Free PMC article.
-
High Molecular Weight Polyproline as a Potential Biosourced Ice Growth Inhibitor: Synthesis, Ice Recrystallization Inhibition, and Specific Ice Face Binding.Biomacromolecules. 2023 Jun 12;24(6):2459-2468. doi: 10.1021/acs.biomac.2c01487. Epub 2023 Feb 21. Biomacromolecules. 2023. PMID: 37303170 Free PMC article.
-
Poly(l-proline)-Stabilized Polypeptide Nanostructures via Ring-Opening Polymerization-Induced Self-Assembly (ROPISA).ACS Macro Lett. 2024 Aug 20;13(8):1031-1036. doi: 10.1021/acsmacrolett.4c00400. Epub 2024 Jul 29. ACS Macro Lett. 2024. PMID: 39074359 Free PMC article.
-
Thermoresponsive Polypeptide Fused L-Asparaginase with Mitigated Immunogenicity and Enhanced Efficacy in Treating Hematologic Malignancies.Adv Sci (Weinh). 2023 Aug;10(23):e2300469. doi: 10.1002/advs.202300469. Epub 2023 Jun 4. Adv Sci (Weinh). 2023. PMID: 37271878 Free PMC article.
-
Open-vessel polymerization of N-carboxyanhydride (NCA) for polypeptide synthesis.Nat Protoc. 2024 Oct 8. doi: 10.1038/s41596-024-01062-3. Online ahead of print. Nat Protoc. 2024. PMID: 39379616 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
