HIV-1 bispecific antibody iMab-N6 exhibits enhanced breadth but not potency over its parental antibodies iMab and N6
- PMID: 36071449
- PMCID: PMC9450465
- DOI: 10.1186/s12985-022-01876-1
HIV-1 bispecific antibody iMab-N6 exhibits enhanced breadth but not potency over its parental antibodies iMab and N6
Abstract
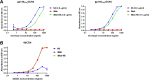
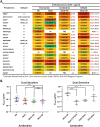
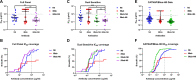
The recently published AMP trial (HVTN 703/HPTN 081 and HVTN704/HPTN 085) results have validated broad neutralising antibodies (bNAbs) as potential anti-HIV-1 agents. However, single bNAb preparations are unlikely to cope with the onslaught of existing and de novo resistance mutations, thus necessitating the use of bNAb combinations to achieve clinically relevant results. Specifically engineered antibodies incorporating two bNAbs into a single antibody structure have been developed. These bispecific antibodies (bibNAbs) retain the benefits of bNAb combinations, whilst several conformations exhibit improved neutralisation potency over the parental bNAbs. Here we report on the engineering of a bibNAb comprising of an HIV-1 spike targeting bNAb N6 and a host CD4 targeting antibody ibalizumab (iMab). Antibodies were expressed in HEK293T cells and purified by protein-A affinity chromatography followed by size exclusion chromatography to achieve homogenous, monomeric, bibNAb preparations. Antibody purity was confirmed by SDS-PAGE whilst epitope specificity and binding were confirmed by ELISA. Finally, antibody breadth and potency data were generated by HIV-1 neutralisation assay (n = 21, inclusive of the global panel). iMab-N6 exhibited better neutralisation breadth (100% coverage) in comparison to its parental bNAbs iMab (90%) and N6 (95%). This is encouraging as exceptional neutralisation breadth is necessary for HIV-1 treatment or prevention. Unfortunately, iMab-N6 did not exhibit any enhancement in potency over the most potent parental antibody, iMab (p = 0.1674, median IC50 of 0.0475 µg/ml, and 0.0665 µg/ml respectively) or the parental combination, iMab + N6 (p = 0.1964, median IC50: combination 0.0457 µg/ml). This result may point to a lack of dual engagement of the bibNAb Fab moieties necessary for potency enhancement. Against the previously reported bibNAbs; iMab-CAP256, 10E08-iMab, and PG9-iMab; iMab-N6 was the lowest performing bibNAb. The re-engineering of iMab-N6 to enhance its potency, while retaining breadth, is a worthwhile endeavour due to its clinical potential.
Keywords: Bispecific antibodies; Bliss-Hill potency prediction; Broadly neutralizing antibodies; HIV-1 prevention; HIV-1 therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Engineering and characterising a novel, highly potent bispecific antibody iMab-CAP256 that targets HIV-1.Retrovirology. 2019 Nov 8;16(1):31. doi: 10.1186/s12977-019-0493-y. Retrovirology. 2019. PMID: 31703699 Free PMC article.
-
Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13540-5. doi: 10.1073/pnas.1304985110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878231 Free PMC article.
-
Rational design and characterization of the novel, broad and potent bispecific HIV-1 neutralizing antibody iMabm36.J Acquir Immune Defic Syndr. 2014 Aug 15;66(5):473-83. doi: 10.1097/QAI.0000000000000218. J Acquir Immune Defic Syndr. 2014. PMID: 24853313 Free PMC article.
-
To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1.Front Immunol. 2021 Oct 19;12:708227. doi: 10.3389/fimmu.2021.708227. eCollection 2021. Front Immunol. 2021. PMID: 34737737 Free PMC article. Review.
-
Divide and conquer: broadly neutralizing antibody combinations for improved HIV-1 viral coverage.Curr Opin HIV AIDS. 2023 Jul 1;18(4):164-170. doi: 10.1097/COH.0000000000000800. Epub 2023 May 19. Curr Opin HIV AIDS. 2023. PMID: 37249911 Free PMC article. Review.
Cited by
-
Broadly neutralizing antibodies for HIV prevention: a comprehensive review and future perspectives.Clin Microbiol Rev. 2024 Jun 13;37(2):e0015222. doi: 10.1128/cmr.00152-22. Epub 2024 Apr 30. Clin Microbiol Rev. 2024. PMID: 38687039 Review.
-
An Overview of Human Anti-HIV-1 Neutralizing Antibodies against Diverse Epitopes of HIV-1.ACS Omega. 2023 Feb 15;8(8):7252-7261. doi: 10.1021/acsomega.2c07933. eCollection 2023 Feb 28. ACS Omega. 2023. PMID: 36873012 Free PMC article. Review.
-
Broadly neutralizing antibodies targeting HIV: Progress and challenges.Clin Immunol. 2023 Dec;257:109809. doi: 10.1016/j.clim.2023.109809. Epub 2023 Oct 16. Clin Immunol. 2023. PMID: 37852345 Free PMC article. Review.
References
-
- Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrovir. 1994;10(4):359–369. doi: 10.1089/aid.1994.10.359. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

