Vaccination with human alphapapillomavirus-derived L2 multimer protects against human betapapillomavirus challenge, including in epidermodysplasia verruciformis model mice
- PMID: 36070626
- PMCID: PMC9710205
- DOI: 10.1016/j.virol.2022.08.006
Vaccination with human alphapapillomavirus-derived L2 multimer protects against human betapapillomavirus challenge, including in epidermodysplasia verruciformis model mice
Abstract
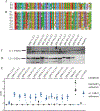
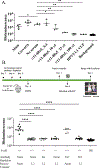
Human alphapapillomaviruses (αHPV) infect genital mucosa, and a high-risk subset is a necessary cause of cervical cancer. Licensed L1 virus-like particle (VLP) vaccines offer immunity against the nine most common αHPV associated with cervical cancer and genital warts. However, vaccination with an αHPV L2-based multimer vaccine, α11-88x5, protected mice and rabbits from vaginal and skin challenge with diverse αHPV types. While generally clinically inapparent, human betapapillomaviruses (βHPV) are possibly associated with cutaneous squamous cell carcinoma (CSCC) in epidermodysplasia verruciformis (EV) and immunocompromised patients. Here we show that α11-88x5 vaccination protected wild type and EV model mice against HPV5 challenge. Passive transfer of antiserum conferred protection independently of Fc receptors (FcR) or Gr-1+ phagocytes. Antisera demonstrated robust antibody titers against ten βHPV by L1/L2 VLP ELISA and neutralized and protected against challenge by 3 additional βHPV (HPV49/76/96). Thus, unlike the licensed vaccines, α11-88x5 vaccination elicits broad immunity against αHPV and βHPV.
Keywords: Cutaneous squamous cell carcinoma; Epidermodysplasia verruciformis; Fc receptor; HPV; Human papillomaviruses; L1 capsid protein; L2 capsid protein; Multimer; Preventive vaccination; Virus-like particle.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Under license agreements between BravoVax Co., Ltd. and the Johns Hopkins University, Dr. Roden is entitled to distributions of payments associated with an invention described in this publication. Dr. Roden also owns equity in PathoVax LLC, Up Therapeutics LLC, Papivax LLC and Papivax Biotech Inc. and is a member of their scientific advisory boards. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures
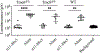

Similar articles
-
RG2-VLP: a Vaccine Designed to Broadly Protect against Anogenital and Skin Human Papillomaviruses Causing Human Cancer.J Virol. 2022 Jul 13;96(13):e0056622. doi: 10.1128/jvi.00566-22. Epub 2022 Jun 15. J Virol. 2022. PMID: 35703545 Free PMC article.
-
Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses.J Invest Dermatol. 2013 Dec;133(12):2706-2713. doi: 10.1038/jid.2013.253. Epub 2013 May 10. J Invest Dermatol. 2013. PMID: 23752042 Free PMC article.
-
Impact of inhibitors and L2 antibodies upon the infectivity of diverse alpha and beta human papillomavirus types.PLoS One. 2014 May 9;9(5):e97232. doi: 10.1371/journal.pone.0097232. eCollection 2014. PLoS One. 2014. PMID: 24816794 Free PMC article.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Virus-like Particle-Based L2 Vaccines against HPVs: Where Are We Today?Viruses. 2019 Dec 23;12(1):18. doi: 10.3390/v12010018. Viruses. 2019. PMID: 31877975 Free PMC article. Review.
Cited by
-
Vaccination with a Human Papillomavirus L2 Multimer Provides Broad Protection against 17 Human Papillomavirus Types in the Mouse Cervicovaginal Challenge Model.Vaccines (Basel). 2024 Jun 20;12(6):689. doi: 10.3390/vaccines12060689. Vaccines (Basel). 2024. PMID: 38932417 Free PMC article.
-
Deficiency in Ever2 does not increase susceptibility of mice to pathogenesis by the mouse papillomavirus, MmuPV1.J Virol. 2024 Jul 23;98(7):e0017424. doi: 10.1128/jvi.00174-24. Epub 2024 Jun 13. J Virol. 2024. PMID: 38869286 Free PMC article.
-
Advances on two serological assays for human papillomavirus provide insights on the reactivity of antibodies against a cross-neutralization epitope of the minor capsid protein L2.Front Immunol. 2023 Nov 8;14:1272018. doi: 10.3389/fimmu.2023.1272018. eCollection 2023. Front Immunol. 2023. PMID: 38022617 Free PMC article.
References
-
- Alam M, Ratner D, 2001. Cutaneous squamous-cell carcinoma. N Engl J Med 344, 975–983. - PubMed
-
- Arroyo Muhr LS, Hultin E, Dillner J, 2021. Transcription of human papillomaviruses in nonmelanoma skin cancers of the immunosuppressed. Int J Cancer 149, 1341–1347. - PubMed
-
- Boey L, Curinckx A, Roelants M, Derdelinckx I, Van Wijngaerden E, De Munter P, Vos R, Kuypers D, Van Cleemput J, Vandermeulen C, 2021. Immunogenicity and Safety of the 9-Valent Human Papillomavirus Vaccine in Solid Organ Transplant Recipients and Adults Infected With Human Immunodeficiency Virus (HIV). Clin Infect Dis 73, e661–e671. - PubMed
-
- Bouwes Bavinck JN, Feltkamp MCW, Green AC, Fiocco M, Euvrard S, Harwood CA, Proby CM, Naldi L, Diphoorn JCD, Venturuzzo A, Tessari G, Nindl I, Sampogna F, Abeni D, Neale RE, Goeman JJ, Quint KD, Halk AB, Sneek C, Genders RE, de Koning MNC, Quint WGV, Wieland U, Weissenborn S, Waterboer T, Pawlita M, Pfister H, group E-H-U-C, 2017. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am J Transplant. - PMC - PubMed

