A replication-competent smallpox vaccine LC16m8Δ-based COVID-19 vaccine
- PMID: 36069348
- PMCID: PMC9527789
- DOI: 10.1080/22221751.2022.2122580
A replication-competent smallpox vaccine LC16m8Δ-based COVID-19 vaccine
Abstract
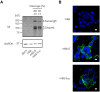
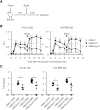
Viral vectors are a potent vaccine platform for inducing humoral and T-cell immune responses. Among the various viral vectors, replication-competent ones are less commonly used for coronavirus disease 2019 (COVID-19) vaccine development compared with replication-deficient ones. Here, we show the availability of a smallpox vaccine LC16m8Δ (m8Δ) as a replication-competent viral vector for a COVID-19 vaccine. M8Δ is a genetically stable variant of the licensed and highly effective Japanese smallpox vaccine LC16m8. Here, we generated two m8Δ recombinants: one harbouring a gene cassette encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) glycoprotein, named m8Δ-SARS2(P7.5-S)-HA; and one encoding the S protein with a highly polybasic motif at the S1/S2 cleavage site, named m8Δ-SARS2(P7.5-SHN)-HA. M8Δ-SARS2(P7.5-S)-HA induced S-specific antibodies in mice that persisted for at least six weeks after a homologous boost immunization. All eight analysed serum samples displayed neutralizing activity against an S-pseudotyped virus at a level similar to that of serum samples from patients with COVID-19, and more than half (5/8) also had neutralizing activity against the Delta/B.1.617.2 variant of concern. Importantly, most serum samples also neutralized the infectious SARS-CoV-2 Wuhan and Delta/B.1.617.2 strains. In contrast, immunization with m8Δ-SARS2(P7.5-SHN)-HA elicited significantly lower antibody titres, and the induced antibodies had less neutralizing activity. Regarding T-cell immunity, both m8Δ recombinants elicited S-specific multifunctional CD8+ and CD4+ T-cell responses even after just a primary immunization. Thus, m8Δ provides an alternative method for developing a novel COVID-19 vaccine.
Keywords: BALB/c mouse; COVID-19; Delta/B.1.617.2 variant of concern; LC16m8; SARS-CoV-2; cellular immunity; emerging and re-Emerging coronaviruses; humoral immunity; prime-boost immunization.
Conflict of interest statement
S.Y., H.S., and M.I. are named inventors on filed patents related to viral-vectored malaria vaccines. H.S. is a named inventor on a filed patent related to m8Δ (WO 2005/054451 A1).
Figures


Similar articles
-
LSDV-Vectored SARS-CoV-2 S and N Vaccine Protects against Severe Clinical Disease in Hamsters.Viruses. 2023 Jun 21;15(7):1409. doi: 10.3390/v15071409. Viruses. 2023. PMID: 37515096 Free PMC article.
-
Single Immunization with Recombinant ACAM2000 Vaccinia Viruses Expressing the Spike and the Nucleocapsid Proteins Protects Hamsters against SARS-CoV-2-Caused Clinical Disease.J Virol. 2022 May 11;96(9):e0038922. doi: 10.1128/jvi.00389-22. Epub 2022 Apr 12. J Virol. 2022. PMID: 35412347 Free PMC article.
-
A Cocktail of Lipid Nanoparticle-mRNA Vaccines Broaden Immune Responses against β-Coronaviruses in a Murine Model.Viruses. 2024 Mar 21;16(3):484. doi: 10.3390/v16030484. Viruses. 2024. PMID: 38543849 Free PMC article.
-
Cross-Protection against MERS-CoV by Prime-Boost Vaccination Using Viral Spike DNA and Protein.J Virol. 2020 Nov 23;94(24):e01176-20. doi: 10.1128/JVI.01176-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32967955 Free PMC article.
-
Adenoviral vector-based platforms for developing effective vaccines to combat respiratory viral infections.Clin Transl Immunology. 2021 Oct 12;10(10):e1345. doi: 10.1002/cti2.1345. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34667600 Free PMC article. Review.
Cited by
-
Long-Term Immunity against SARS-CoV-2 Wild-Type and Omicron XBB.1.5 in Indonesian Residents after Vaccination and Infection.Antibodies (Basel). 2024 Sep 2;13(3):72. doi: 10.3390/antib13030072. Antibodies (Basel). 2024. PMID: 39311377 Free PMC article.
-
Comparison between Neutralization Capacity of Antibodies Elicited by COVID-19 Natural Infection and Vaccination in Indonesia: A Prospective Cohort.Antibodies (Basel). 2023 Sep 21;12(3):60. doi: 10.3390/antib12030060. Antibodies (Basel). 2023. PMID: 37753974 Free PMC article.
-
Indirect Dispersion of SARS-CoV-2 Live-Attenuated Vaccine and Its Contribution to Herd Immunity.Vaccines (Basel). 2023 Mar 14;11(3):655. doi: 10.3390/vaccines11030655. Vaccines (Basel). 2023. PMID: 36992239 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
