Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischemia
- PMID: 36068595
- PMCID: PMC9449296
- DOI: 10.1186/s13287-022-03148-9
Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischemia
Abstract
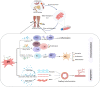
Critical limb ischemia (CLI), the terminal stage of peripheral arterial disease (PAD), is characterized by an extremely high risk of amputation and vascular issues, resulting in severe morbidity and mortality. In patients with severe limb ischemia with no alternative therapy options, such as endovascular angioplasty or bypass surgery, therapeutic angiogenesis utilizing cell-based therapies is vital for increasing blood flow to ischemic regions. Mesenchymal stem cells (MSCs) are currently considered one of the most encouraging cells as a regenerative alternative for the surgical treatment of CLI, including restoring tissue function and repairing ischemic tissue via immunomodulation and angiogenesis. The regenerative treatments for limb ischemia based on MSC therapy are still considered experimental. Despite recent advances in preclinical and clinical research studies, it is not recommended for regular clinical use. In this study, we review the immunomodulatory features of MSC besides the current understanding of different sources of MSC in the angiogenic treatment of CLI subjects and their potential applications as therapeutic agents. Specifically, this paper concentrates on the most current clinical application issues, and several recommendations are provided to improve the efficacy of cell therapy for CLI patients.
Keywords: Angiogenesis; Cell therapy; Clinical trials; Critical limb ischemia; Hindlimb ischemia; Mesenchymal stem cells; Peripheral arterial disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy.Stem Cell Res Ther. 2021 Jan 13;12(1):58. doi: 10.1186/s13287-020-02110-x. Stem Cell Res Ther. 2021. PMID: 33436054 Free PMC article.
-
Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with critical limb ischemia (CLI).Stem Cell Res Ther. 2023 Jul 5;14(1):174. doi: 10.1186/s13287-023-03390-9. Stem Cell Res Ther. 2023. PMID: 37408043 Free PMC article. Clinical Trial.
-
Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model.Acta Biomater. 2019 Feb;85:94-105. doi: 10.1016/j.actbio.2018.12.015. Epub 2018 Dec 11. Acta Biomater. 2019. PMID: 30550934
-
Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia.Stem Cell Res Ther. 2012 Jul 30;3(4):28. doi: 10.1186/scrt119. Stem Cell Res Ther. 2012. PMID: 22846185 Free PMC article. Review.
-
Mesenchymal stem cells for critical limb ischemia: their function, mechanism, and therapeutic potential.Stem Cell Res Ther. 2022 Jul 26;13(1):345. doi: 10.1186/s13287-022-03043-3. Stem Cell Res Ther. 2022. PMID: 35883198 Free PMC article. Review.
Cited by
-
The Use of Autologous Cell Therapy in Diabetic Patients with Chronic Limb-Threatening Ischemia.Int J Mol Sci. 2024 Sep 23;25(18):10184. doi: 10.3390/ijms251810184. Int J Mol Sci. 2024. PMID: 39337669 Free PMC article. Review.
-
Effect of acidosis on adipose-derived stem cell impairment and gene expression.Regen Ther. 2024 Feb 5;25:331-343. doi: 10.1016/j.reth.2024.01.010. eCollection 2024 Mar. Regen Ther. 2024. PMID: 38333090 Free PMC article.
-
Large extracellular vesicles from induced pluripotent stem cell-marrow stem cells enhance limb angiogenesis via ERK/MAPK.Nanomedicine (Lond). 2024 Jul 14;19(17):1525-1539. doi: 10.1080/17435889.2024.2363743. Epub 2024 Jul 16. Nanomedicine (Lond). 2024. PMID: 39012207 Free PMC article.
-
A novel therapeutic management for diabetes patients with chronic limb-threatening ischemia: comparison of autologous bone marrow mononuclear cells versus allogenic Wharton jelly-derived mesenchymal stem cells.Stem Cell Res Ther. 2023 Aug 25;14(1):221. doi: 10.1186/s13287-023-03427-z. Stem Cell Res Ther. 2023. PMID: 37626416 Free PMC article. Clinical Trial.
-
Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin.Pharmaceutics. 2023 Mar 24;15(4):1049. doi: 10.3390/pharmaceutics15041049. Pharmaceutics. 2023. PMID: 37111534 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

