Truncated Tau caused by intron retention is enriched in Alzheimer's disease cortex and exhibits altered biochemical properties
- PMID: 36067305
- PMCID: PMC9477417
- DOI: 10.1073/pnas.2204179119
Truncated Tau caused by intron retention is enriched in Alzheimer's disease cortex and exhibits altered biochemical properties
Abstract
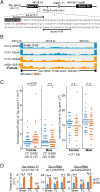
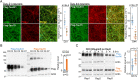
Alzheimer's disease (AD) is characterized by the accumulation of amyloid-β plaques and Tau tangles in brain tissues. Recent studies indicate that aberrant splicing and increased level of intron retention is linked to AD pathogenesis. Bioinformatic analysis revealed increased retention of intron 11 at the Tau gene in AD female dorsal lateral prefrontal cortex as compared to healthy controls, an observation validated by quantitative polymerase chain reaction using different brain tissues. Retention of intron 11 introduces a premature stop codon, resulting in the production of truncated Tau11i protein. Probing with customized antibodies designed against amino acids encoded by intron 11 showed that Tau11i protein is more enriched in AD hippocampus, amygdala, parietal, temporal, and frontal lobe than in healthy controls. This indicates that Tau messenger RNA with the retained intron is translated in vivo instead of being subjected to nonsense-mediated decay. Compared to full-length Tau441 isoform, ectopically expressed Tau11i forms higher molecular weight species, is enriched in Sarkosyl-insoluble fraction, and exhibits greater protein stability in cycloheximide assay. Stably expressed Tau11i also shows weaker colocalization with α-tubulin of microtubule network in human mature cortical neurons as compared to Tau441. Endogenous Tau11i is enriched in Sarkosyl-insoluble fraction in AD hippocampus and forms aggregates that colocalize weakly with Tau4R fibril-like structure in AD temporal lobe. The elevated level of Tau11i protein in AD brain tissues tested, coupled with biochemical properties resembling pathological Tau species suggest that retention of intron 11 of Tau gene might be an early biomarker of AD pathology.
Keywords: Alzheimer disease; Tau; intron retention.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Intron retention as a productive mechanism in human MAPT: RNA species generated by retention of intron 3.EBioMedicine. 2024 Feb;100:104953. doi: 10.1016/j.ebiom.2023.104953. Epub 2024 Jan 5. EBioMedicine. 2024. PMID: 38181704 Free PMC article.
-
Quantitative proteomics of tau and Aβ in detergent fractions from Alzheimer's disease brains.J Neurochem. 2023 Feb;164(4):529-552. doi: 10.1111/jnc.15713. Epub 2022 Nov 22. J Neurochem. 2023. PMID: 36271678
-
Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis.JAMA Neurol. 2014 Apr;71(4):505-8. doi: 10.1001/jamaneurol.2013.5847. JAMA Neurol. 2014. PMID: 24493463 Review.
-
A new non-aggregative splicing isoform of human Tau is decreased in Alzheimer's disease.Acta Neuropathol. 2021 Jul;142(1):159-177. doi: 10.1007/s00401-021-02317-z. Epub 2021 May 2. Acta Neuropathol. 2021. PMID: 33934221 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Automatic detection of Alzheimer's disease from EEG signals using an improved AFS-GA hybrid algorithm.Cogn Neurodyn. 2024 Oct;18(5):2993-3013. doi: 10.1007/s11571-024-10130-z. Epub 2024 Jun 10. Cogn Neurodyn. 2024. PMID: 39555281
-
Nuclear face of Tau: an inside player in neurodegeneration.Acta Neuropathol Commun. 2023 Dec 12;11(1):196. doi: 10.1186/s40478-023-01702-x. Acta Neuropathol Commun. 2023. PMID: 38087392 Free PMC article. Review.
-
Enrichment of novel Tau isoform with altered biochemical properties in Alzheimer's disease.Neural Regen Res. 2023 Nov;18(11):2397-2398. doi: 10.4103/1673-5374.371359. Neural Regen Res. 2023. PMID: 37282466 Free PMC article. No abstract available.
-
ApoE maintains neuronal integrity via microRNA and H3K27me3-mediated repression.iScience. 2024 Feb 15;27(3):109231. doi: 10.1016/j.isci.2024.109231. eCollection 2024 Mar 15. iScience. 2024. PMID: 38439966 Free PMC article.
-
Post-transcriptional Regulation of Gene Expression via Unproductive Splicing.Acta Naturae. 2024 Jan-Mar;16(1):4-13. doi: 10.32607/actanaturae.27337. Acta Naturae. 2024. PMID: 38698955 Free PMC article.
References
-
- Sims R., Hill M., Williams J., The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 23, 311–322 (2020). - PubMed
-
- Smith A. R., Wheildon G., Lunnon K., Invited review—A 5-year update on epigenome-wide association studies of DNA modifications in Alzheimer’s disease: Progress, practicalities and promise. Neuropathol. Appl. Neurobiol. 46, 641–653 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

