Exploration of beta-arrestin isoform signaling pathways in delta opioid receptor agonist-induced convulsions
- PMID: 36059958
- PMCID: PMC9428791
- DOI: 10.3389/fphar.2022.914651
Exploration of beta-arrestin isoform signaling pathways in delta opioid receptor agonist-induced convulsions
Abstract
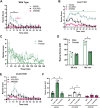
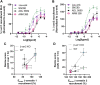
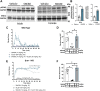
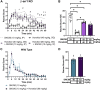
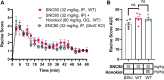
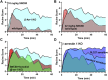
The δ-opioid receptor (δOR) has been considered as a therapeutic target in multiple neurological and neuropsychiatric disorders particularly as δOR agonists are deemed safer alternatives relative to the more abuse-liable µ-opioid receptor drugs. Clinical development of δOR agonists, however, has been challenging in part due to the seizure-inducing effects of certain δOR agonists. Especially agonists that resemble the δOR-selective agonist SNC80 have well-established convulsive activity. Close inspection suggests that many of those seizurogenic δOR agonists efficaciously recruit β-arrestin, yet surprisingly, SNC80 displays enhanced seizure activity in β-arrestin 1 knockout mice. This finding led us to hypothesize that perhaps β-arrestin 1 is protective against, whereas β-arrestin 2 is detrimental for δOR-agonist-induced seizures. To investigate our hypothesis, we characterized three different δOR agonists (SNC80, ADL5859, ARM390) in cellular assays and in vivo in wild-type and β-arrestin 1 and β-arrestin 2 knockout mice for seizure activity. We also investigated downstream kinases associated with β-arrestin-dependent signal transduction. We discovered that δOR agonist-induced seizure activity strongly and positively correlates with β-arrestin 2 efficacy for the agonist, but that indirect inhibition of ERK activation using the MEK inhibitor SL327 did not inhibit seizure potency and duration. Inhibition of the PI3K/AKT/mTOR signaling with honokiol but not PQR530, attenuated SNC80 seizure duration in β-arrestin 1 knockout, but honokiol did not reduce SNC80-induced seizures in wild-type mice. Ultimately, our results indicate that β-arrestin 2 is correlated with δOR agonist-induced seizure intensity, but that global β-arrestin 1 knockout mice are a poor model system to investigate their mechanism of action.
Keywords: ERK; PQR530; beta-arrestin 1; beta-arrestin 2; biased signaling; honokiol; mice; seizure.
Copyright © 2022 Blaine, Miao, Yuan, Palant, Liu, Zhang and van Rijn.
Conflict of interest statement
RVR is currently employed as a Principal Scientist at Septerna Inc. and holds stock options in the company. RVR holds a US patent (10,954,224) describing novel delta opioid receptor agonists. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Receptor expression and signaling properties in the brain, and structural ligand motifs that contribute to delta opioid receptor agonist-induced seizures.Neuropharmacology. 2023 Jul 1;232:109526. doi: 10.1016/j.neuropharm.2023.109526. Epub 2023 Mar 31. Neuropharmacology. 2023. PMID: 37004753 Free PMC article. Review.
-
Agonist-Specific Recruitment of Arrestin Isoforms Differentially Modify Delta Opioid Receptor Function.J Neurosci. 2016 Mar 23;36(12):3541-51. doi: 10.1523/JNEUROSCI.4124-15.2016. J Neurosci. 2016. PMID: 27013682 Free PMC article.
-
Identification of a Novel Delta Opioid Receptor Agonist Chemotype with Potential Negative Allosteric Modulator Capabilities.Molecules. 2021 Nov 29;26(23):7236. doi: 10.3390/molecules26237236. Molecules. 2021. PMID: 34885825 Free PMC article.
-
Tolerance to high-internalizing δ opioid receptor agonist is critically mediated by arrestin 2.Br J Pharmacol. 2018 Jul;175(14):3050-3059. doi: 10.1111/bph.14353. Epub 2018 Jun 7. Br J Pharmacol. 2018. PMID: 29722902 Free PMC article.
-
Ligand-Directed Signaling at the Delta Opioid Receptor.Handb Exp Pharmacol. 2018;247:73-85. doi: 10.1007/164_2017_39. Handb Exp Pharmacol. 2018. PMID: 28689302 Review.
Cited by
-
ClickArr: a novel, high-throughput assay for evaluating β-arrestin isoform recruitment.Front Pharmacol. 2023 Nov 7;14:1295518. doi: 10.3389/fphar.2023.1295518. eCollection 2023. Front Pharmacol. 2023. PMID: 38027002 Free PMC article.
-
Receptor expression and signaling properties in the brain, and structural ligand motifs that contribute to delta opioid receptor agonist-induced seizures.Neuropharmacology. 2023 Jul 1;232:109526. doi: 10.1016/j.neuropharm.2023.109526. Epub 2023 Mar 31. Neuropharmacology. 2023. PMID: 37004753 Free PMC article. Review.
-
Identification of 1,3,8-Triazaspiro[4.5]Decane-2,4-Dione Derivatives as a Novel δ Opioid Receptor-Selective Agonist Chemotype.J Pharmacol Exp Ther. 2024 May 21;389(3):301-309. doi: 10.1124/jpet.123.001735. J Pharmacol Exp Ther. 2024. PMID: 38621994
References
-
- Alongkronrusmee D., Chiang T., Van Rijn R. M. (2018). Delta opioid pharmacology in relation to alcohol behaviors. Handb. Exp. Pharmacol. 247, 199–225. 10.1007/164_2016_30 PubMed Abstract | 10.1007/164_2016_30 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Bai X., Cerimele F., Ushio-Fukai M., Waqas M., Campbell P. M., Govindarajan B., et al. (2003). Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo . J. Biol. Chem. 278, 35501–35507. 10.1074/jbc.M302967200 PubMed Abstract | 10.1074/jbc.M302967200 | Google Scholar - DOI - DOI - PubMed
-
- Bausch S. B., Garland J. P., Yamada J. (2005). The delta opioid receptor agonist, SNC80, has complex, dose-dependent effects on pilocarpine-induced seizures in Sprague-Dawley rats. Brain Res. 1045, 38–44. 10.1016/j.brainres.2005.03.008 PubMed Abstract | 10.1016/j.brainres.2005.03.008 | Google Scholar - DOI - DOI - PubMed
-
- Bosse K. E., Jutkiewicz E. M., Schultz-Kuszak K. N., Mabrouk O. S., Kennedy R. T., Gnegy M. E., et al. (2014). Synergistic activity between the delta-opioid agonist SNC80 and amphetamine occurs via a glutamatergic NMDA-receptor dependent mechanism. Neuropharmacology 77, 19–27. 10.1016/j.neuropharm.2013.08.027 PubMed Abstract | 10.1016/j.neuropharm.2013.08.027 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Brandt C., Hillmann P., Noack A., Romermann K., Ohler L. A., Rageot D., et al. (2018). The novel, catalytic mTORC1/2 inhibitor PQR620 and the PI3K/mTORC1/2 inhibitor PQR530 effectively cross the blood-brain barrier and increase seizure threshold in a mouse model of chronic epilepsy. Neuropharmacology 140, 107–120. 10.1016/j.neuropharm.2018.08.002 PubMed Abstract | 10.1016/j.neuropharm.2018.08.002 | Google Scholar - DOI - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

