Stem-like T cells and niches: Implications in human health and disease
- PMID: 36059484
- PMCID: PMC9428355
- DOI: 10.3389/fimmu.2022.907172
Stem-like T cells and niches: Implications in human health and disease
Abstract
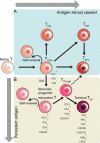
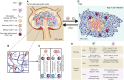
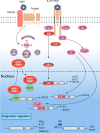
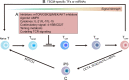
Recently, accumulating evidence has elucidated the important role of T cells with stem-like characteristics in long-term maintenance of T cell responses and better patient outcomes after immunotherapy. The fate of TSL cells has been correlated with many physiological and pathological human processes. In this review, we described present advances demonstrating that stem-like T (TSL) cells are central players in human health and disease. We interpreted the evolutionary characteristics, mechanism and functions of TSL cells. Moreover, we discuss the import role of distinct niches and how they affect the stemness of TSL cells. Furthermore, we also outlined currently available strategies to generate TSL cells and associated affecting factors. Moreover, we summarized implication of TSL cells in therapies in two areas: stemness enhancement for vaccines, ICB, and adoptive T cell therapies, and stemness disruption for autoimmune disorders.
Keywords: adoptive cell therapies; autoimmune disorders; immune checkpoint blockade; niches; stem-like T cells.
Copyright © 2022 Yi and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Stem-like CD8+ T cells in cancer.Front Immunol. 2024 Aug 15;15:1426418. doi: 10.3389/fimmu.2024.1426418. eCollection 2024. Front Immunol. 2024. PMID: 39211052 Free PMC article. Review.
-
T memory stem cells in health and disease.Nat Med. 2017 Jan 6;23(1):18-27. doi: 10.1038/nm.4241. Nat Med. 2017. PMID: 28060797 Free PMC article. Review.
-
IL15 and T-cell Stemness in T-cell-Based Cancer Immunotherapy.Cancer Res. 2015 Dec 15;75(24):5187-5193. doi: 10.1158/0008-5472.CAN-15-1498. Epub 2015 Dec 1. Cancer Res. 2015. PMID: 26627006 Free PMC article. Review.
-
Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies.Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(1):68-77. doi: 10.2177/jsci.40.68. Nihon Rinsho Meneki Gakkai Kaishi. 2017. PMID: 28539557 Review.
-
Pathogenesis of age dependent paralysis by a temperature sensitive mutant (tsl) of Moloney murine leukemia virus-TB.Indian J Exp Biol. 1992 Sep;30(9):814-8. Indian J Exp Biol. 1992. PMID: 1478716
Cited by
-
T-cell exhaustion and stemness in antitumor immunity: Characteristics, mechanisms, and implications.Front Immunol. 2023 Feb 20;14:1104771. doi: 10.3389/fimmu.2023.1104771. eCollection 2023. Front Immunol. 2023. PMID: 36891319 Free PMC article. Review.
-
Postinfusion PD-1+ CD8+ CAR T cells identify patients responsive to CD19 CAR T-cell therapy in non-Hodgkin lymphoma.Blood Adv. 2024 Jun 25;8(12):3140-3153. doi: 10.1182/bloodadvances.2023012073. Blood Adv. 2024. PMID: 38607381 Free PMC article.
-
Stem-like T cells are associated with the pathogenesis of ulcerative colitis in humans.Nat Immunol. 2024 Jul;25(7):1231-1244. doi: 10.1038/s41590-024-01860-7. Epub 2024 Jun 19. Nat Immunol. 2024. PMID: 38898157
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

