Hypoxic glioma-derived extracellular vesicles harboring MicroRNA-10b-5p enhance M2 polarization of macrophages to promote the development of glioma
- PMID: 36052751
- PMCID: PMC9532931
- DOI: 10.1111/cns.13905
Hypoxic glioma-derived extracellular vesicles harboring MicroRNA-10b-5p enhance M2 polarization of macrophages to promote the development of glioma
Abstract
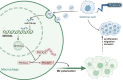
Introduction: The delivery of biomolecules by tumor cell-secreted extracellular vesicles (EVs) is linked to the development of glioma. Here, the present study was implemented to explore the functional significance of hypoxic glioma cell-derived EVs carrying microRNA-10b-5 (miR-10b-5p) on glioma with the involvement of polarization of M2 macrophages.
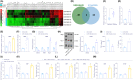
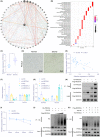
Methods: EVs were isolated from hypoxia-stimulated glioma cells, and their role in polarization of M2 macrophages was studied by co-culturing with macrophages. miR-10b-5p expression in glioma tissues, glioma-derived EVs, and macrophages co-cultured with EVs was characterized. Interaction among miR-10b-5p, NEDD4L, and PIK3CA was analyzed. The macrophages or glioma cells were transfected with overexpressing plasmid or shRNA to study the effects of miR-10b-5p/NEDD4L/PIK3CA on M2 macrophage polarization, and glioma cell proliferation, migration, and invasion in vitro and in vivo.
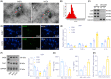
Results: Promotive role of hypoxia-stimulated glioma-derived EVs in macrophage M2 polarization was confirmed. Elevation of miR-10b-5p occurred in glioma tissues, glioma-derived EVs and macrophages co-cultured with EVs, and stimulated M2 polarization of macrophages. NEDD4L was a target gene of miR-10b-5p. Overexpression of NEDD4L could inhibit PI3K/AKT pathway through increase in ubiquitination and degradation of PIK3CA. Hypoxic glioma-derived EVs harboring upregulated miR-10b-5p triggered an M2 phenotype in macrophages as well as enhanced aggressive tumor biology of glioma cells via inhibition of PIK3CA/PI3K/AKT pathway by targeting NEDD4L.
Conclusions: In summary, miR-10b-5p delivered by hypoxic glioma-derived EVs accelerated macrophages M2 polarization to promote the progression of glioma via NEDD4L/PIK3CA/PI3K/AKT axis.
Keywords: extracellular vesicles; glioma; microRNA-10b-5; neuronal precursor cell expressed developmentally downregulated 4-like; phosphatidylinositol-4, 5-bisphosphate 3-kinase.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Extracellular Vesicles Derived from Glioma Stem Cells Affect Glycometabolic Reprogramming of Glioma Cells Through the miR-10b-5p/PTEN/PI3K/Akt Pathway.Stem Cell Rev Rep. 2024 Apr;20(3):779-796. doi: 10.1007/s12015-024-10677-8. Epub 2024 Jan 31. Stem Cell Rev Rep. 2024. PMID: 38294721
-
Extracellular vesicle-packaged miR-181c-5p from epithelial ovarian cancer cells promotes M2 polarization of tumor-associated macrophages via the KAT2B/HOXA10 axis.J Gene Med. 2022 Oct;24(10):e3446. doi: 10.1002/jgm.3446. Epub 2022 Sep 19. J Gene Med. 2022. PMID: 36027869
-
Mesenchymal stem cell-derived extracellular vesicles prevent glioma by blocking M2 polarization of macrophages through a miR-744-5p/TGFB1-dependent mechanism.Cell Biol Toxicol. 2022 Aug;38(4):649-665. doi: 10.1007/s10565-021-09652-7. Epub 2022 Jan 3. Cell Biol Toxicol. 2022. PMID: 34978010
-
Tumor-derived extracellular vesicles: how they mediate glioma immunosuppression.Mol Biol Rep. 2024 Jan 28;51(1):235. doi: 10.1007/s11033-023-09196-5. Mol Biol Rep. 2024. PMID: 38282090 Review.
-
Microglia and Brain Macrophages as Drivers of Glioma Progression.Int J Mol Sci. 2022 Dec 9;23(24):15612. doi: 10.3390/ijms232415612. Int J Mol Sci. 2022. PMID: 36555253 Free PMC article. Review.
Cited by
-
Sustained Effectiveness and Safety of Therapeutic miR-10a/b in Alleviating Diabetes and Gastrointestinal Dysmotility without Inducing Cancer or Inflammation in Murine Liver and Colon.Int J Mol Sci. 2024 Feb 14;25(4):2266. doi: 10.3390/ijms25042266. Int J Mol Sci. 2024. PMID: 38396943 Free PMC article.
-
Microglial Extracellular Vesicles as Modulators of Brain Microenvironment in Glioma.Int J Mol Sci. 2022 Oct 29;23(21):13165. doi: 10.3390/ijms232113165. Int J Mol Sci. 2022. PMID: 36361947 Free PMC article. Review.
-
Prognostic and immune implications of a novel 7-methylguanosine-related microRNA signature in breast invasive carcinoma: from exploration to validation.J Cancer Res Clin Oncol. 2023 Sep;149(11):9105-9128. doi: 10.1007/s00432-023-04849-1. Epub 2023 May 12. J Cancer Res Clin Oncol. 2023. PMID: 37171615
-
NEDD4L in human tumors: regulatory mechanisms and dual effects on anti-tumor and pro-tumor.Front Pharmacol. 2023 Nov 9;14:1291773. doi: 10.3389/fphar.2023.1291773. eCollection 2023. Front Pharmacol. 2023. PMID: 38027016 Free PMC article. Review.
-
The negative effects of extracellular vesicles in the immune system.Front Immunol. 2024 Sep 20;15:1410273. doi: 10.3389/fimmu.2024.1410273. eCollection 2024. Front Immunol. 2024. PMID: 39372421 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

