Efficacy of Human Embryonic Stem Cells Compared to Adipose Tissue-Derived Human Mesenchymal Stem/Stromal Cells for Repair of Murine Post-Stenotic Kidneys
- PMID: 36048327
- PMCID: PMC9905277
- DOI: 10.1007/s12015-022-10443-8
Efficacy of Human Embryonic Stem Cells Compared to Adipose Tissue-Derived Human Mesenchymal Stem/Stromal Cells for Repair of Murine Post-Stenotic Kidneys
Abstract
Clinical translation of mesenchymal stem/stromal cell (MSC) therapy has been impeded by the heterogenous nature and limited replicative potential of adult-derived MSCs. Human embryonic stem cell-derived MSCs (hESC-MSCs) that differentiate from immortal cell lines are phenotypically uniform and have shown promise in-vitro and in many disease models. Similarly, adipose tissue-derived MSCs (MSC(AT)) possess potent reparative properties. How these two cell types compare in efficacy, however, remains unknown. We randomly assigned mice to six groups (n = 7-8 each) that underwent unilateral RAS or a sham procedure (3 groups each). Two weeks post-operation, each mouse was administered either vehicle, MSC(AT)s, or hESC-MSCs (5 × 105 cells) into the aorta. Mice were scanned with micro-MRI to determine renal hemodynamics two weeks later and kidneys then harvested. hESC-MSCs and MSC(AT)s were similarly effective at lowering systolic blood pressure. However, MSC(AT)s more robustly increased renal perfusion, oxygenation, and glomerular filtration rate in the post-stenotic kidney, and more effectively mitigated tubular injury, fibrosis, and vascular remodeling. These observations suggest that MSC(AT) are more effective than hESC-MSC in ameliorating kidney dysfunction and tissue injury distal to RAS. Our findings highlight the importance of tissue source in selection of MSCs for therapeutic purposes and underscore the utility of cell-based therapy for kidney disease.
Keywords: mesenchymal stem/stromal cells; mouse; renal artery stenosis.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflicts of Interest
Dr. Lerman is an advisor to AstraZeneca, CureSpec, and Butterfly Biosciences. The authors declare no conflict.
Figures

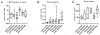
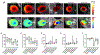
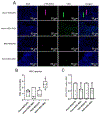
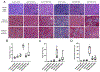

Similar articles
-
Effects of obesity on reparative function of human adipose tissue-derived mesenchymal stem cells on ischemic murine kidneys.Int J Obes (Lond). 2022 Jun;46(6):1222-1233. doi: 10.1038/s41366-022-01103-5. Epub 2022 Mar 7. Int J Obes (Lond). 2022. PMID: 35256761 Free PMC article.
-
Selective kidney targeting increases the efficacy of mesenchymal stromal/stem cells for alleviation of murine stenotic-kidney senescence and damage.J Tissue Eng Regen Med. 2022 Jun;16(6):550-558. doi: 10.1002/term.3299. Epub 2022 Mar 23. J Tissue Eng Regen Med. 2022. PMID: 35319825 Free PMC article.
-
Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension.Cell Transplant. 2012;21(10):2225-39. doi: 10.3727/096368912X653020. Epub 2012 Jul 5. Cell Transplant. 2012. PMID: 22776744 Clinical Trial.
-
Cell-based regenerative medicine for renovascular disease.Trends Mol Med. 2021 Sep;27(9):882-894. doi: 10.1016/j.molmed.2021.06.004. Epub 2021 Jun 25. Trends Mol Med. 2021. PMID: 34183258 Free PMC article. Review.
-
Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models.Front Med (Lausanne). 2018 Jun 15;5:179. doi: 10.3389/fmed.2018.00179. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29963554 Free PMC article. Review.
Cited by
-
The Role of Dental-derived Stem Cell-based Therapy and Their Derived Extracellular Vesicles in Post-COVID-19 Syndrome-induced Tissue Damage.Stem Cell Rev Rep. 2024 Nov;20(8):2062-2103. doi: 10.1007/s12015-024-10770-y. Epub 2024 Aug 16. Stem Cell Rev Rep. 2024. PMID: 39150646 Review.
References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, … Wright JT Jr (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 71(19), e127–e248. - PubMed
-
- Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, & Grande JP (2010). Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 25(11), 3615–3622. - PMC - PubMed
-
- Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, & Textor SC (2013). Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circulation. Cardiovascular interventions, 6(4), 428–435. - PMC - PubMed
-
- Kim SR, Eirin A, Zhang X, Lerman A, & Lerman LO (2019). Mitochondrial Protection Partly Mitigates Kidney Cellular Senescence in Swine Atherosclerotic Renal Artery Stenosis. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 52(3), 617–632. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

