Mid51/Fis1 mitochondrial oligomerization complex drives lysosomal untethering and network dynamics
- PMID: 36044022
- PMCID: PMC9437119
- DOI: 10.1083/jcb.202206140
Mid51/Fis1 mitochondrial oligomerization complex drives lysosomal untethering and network dynamics
Abstract
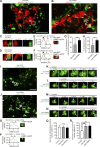
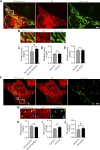
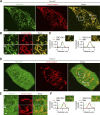
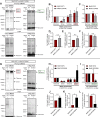
Lysosomes are highly dynamic organelles implicated in multiple diseases. Using live super-resolution microscopy, we found that lysosomal tethering events rarely undergo lysosomal fusion, but rather untether over time to reorganize the lysosomal network. Inter-lysosomal untethering events are driven by a mitochondrial Mid51/Fis1 complex that undergoes coupled oligomerization on the outer mitochondrial membrane. Importantly, Fis1 oligomerization mediates TBC1D15 (Rab7-GAP) mitochondrial recruitment to drive inter-lysosomal untethering via Rab7 GTP hydrolysis. Moreover, inhibiting Fis1 oligomerization by either mutant Fis1 or a Mid51 oligomerization mutant potentially associated with Parkinson's disease prevents lysosomal untethering events, resulting in misregulated lysosomal network dynamics. In contrast, dominant optic atrophy-linked mutant Mid51, which does not inhibit Mid51/Fis1 coupled oligomerization, does not disrupt downstream lysosomal dynamics. As Fis1 conversely also regulates Mid51 oligomerization, our work further highlights an oligomeric Mid51/Fis1 mitochondrial complex that mechanistically couples together both Drp1 and Rab7 GTP hydrolysis machinery at mitochondria-lysosome contact sites. These findings have significant implications for organelle networks in cellular homeostasis and human disease.
© 2022 Wong et al.
Figures

Similar articles
-
Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission.J Biol Chem. 2013 Sep 20;288(38):27584-27593. doi: 10.1074/jbc.M113.479873. Epub 2013 Aug 6. J Biol Chem. 2013. PMID: 23921378 Free PMC article.
-
Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis.Nature. 2018 Feb 15;554(7692):382-386. doi: 10.1038/nature25486. Epub 2018 Jan 24. Nature. 2018. PMID: 29364868 Free PMC article.
-
Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation-induced apoptosis.FASEB J. 2016 Jan;30(1):466-76. doi: 10.1096/fj.15-274258. Epub 2015 Oct 2. FASEB J. 2016. PMID: 26432782
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
-
The role of Mitochondrial Fission Proteins in Mitochondrial Dynamics in Kidney Disease.Int J Mol Sci. 2022 Nov 25;23(23):14725. doi: 10.3390/ijms232314725. Int J Mol Sci. 2022. PMID: 36499050 Free PMC article. Review.
Cited by
-
The mystery of phospho-Drp1 with four adaptors in cell cycle: when mitochondrial fission couples to cell fate decisions.Cell Cycle. 2023 Nov;22(21-22):2485-2503. doi: 10.1080/15384101.2023.2289753. Epub 2024 Jan 18. Cell Cycle. 2023. PMID: 38053243 Free PMC article.
-
Multifaceted functions of Drp1 in hypoxia/ischemia-induced mitochondrial quality imbalance: from regulatory mechanism to targeted therapeutic strategy.Mil Med Res. 2023 Oct 13;10(1):46. doi: 10.1186/s40779-023-00482-8. Mil Med Res. 2023. PMID: 37833768 Free PMC article. Review.
-
Parkin regulates amino acid homeostasis at mitochondria-lysosome (M/L) contact sites in Parkinson's disease.Sci Adv. 2023 Jul 21;9(29):eadh3347. doi: 10.1126/sciadv.adh3347. Epub 2023 Jul 19. Sci Adv. 2023. PMID: 37467322 Free PMC article.
-
Mitochondrial dysfunction induced by HIF-1α under hypoxia contributes to the development of gastric mucosal lesions.Clin Transl Med. 2024 Apr;14(4):e1653. doi: 10.1002/ctm2.1653. Clin Transl Med. 2024. PMID: 38616702 Free PMC article.
-
Hypoxia-reprogramed megamitochondrion contacts and engulfs lysosome to mediate mitochondrial self-digestion.Nat Commun. 2023 Jul 11;14(1):4105. doi: 10.1038/s41467-023-39811-9. Nat Commun. 2023. PMID: 37433770 Free PMC article.
References
-
- Charif, M., Wong Y.C., Kim S., Guichet A., Vignal C., Zanlonghi X., Bensaid P., Procaccio V., Bonneau D., Amati-Bonneau P., et al. . 2021. Dominant mutations in MIEF1 affect mitochondrial dynamics and cause a singular late onset optic neuropathy. Mol. Neurodegener. 16:12. 10.1186/s13024-021-00431-w - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

