Early life sleep disruption potentiates lasting sex-specific changes in behavior in genetically vulnerable Shank3 heterozygous autism model mice
- PMID: 36038911
- PMCID: PMC9425965
- DOI: 10.1186/s13229-022-00514-5
Early life sleep disruption potentiates lasting sex-specific changes in behavior in genetically vulnerable Shank3 heterozygous autism model mice
Abstract
Background: Patients with autism spectrum disorder (ASD) experience high rates of sleep disruption beginning early in life; however, the developmental consequences of this disruption are not understood. We examined sleep behavior and the consequences of sleep disruption in developing mice bearing C-terminal truncation mutation in the high-confidence ASD risk gene SHANK3 (Shank3ΔC). We hypothesized that sleep disruption may be an early sign of developmental divergence, and that clinically relevant Shank3WT/ΔC mice may be at increased risk of lasting deleterious outcomes following early life sleep disruption.
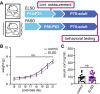
Methods: We recorded sleep behavior in developing Shank3ΔC/ΔC, Shank3WT/ΔC, and wild-type siblings of both sexes using a noninvasive home-cage monitoring system. Separately, litters of Shank3WT/ΔC and wild-type littermates were exposed to automated mechanical sleep disruption for 7 days prior to weaning (early life sleep disruption: ELSD) or post-adolescence (PASD) or undisturbed control (CON) conditions. All groups underwent standard behavioral testing as adults.
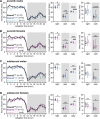
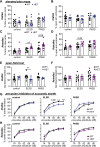
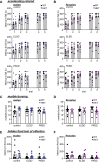
Results: Male and female Shank3ΔC/ΔC mice slept significantly less than wild-type and Shank3WT/ΔC siblings shortly after weaning, with increasing sleep fragmentation in adolescence, indicating that sleep disruption has a developmental onset in this ASD model. ELSD treatment interacted with genetic vulnerability in Shank3WT/ΔC mice, resulting in lasting, sex-specific changes in behavior, whereas wild-type siblings were largely resilient to these effects. Male ELSD Shank3WT/ΔC subjects demonstrated significant changes in sociability, sensory processing, and locomotion, while female ELSD Shank3WT/ΔC subjects had a significant reduction in risk aversion. CON Shank3WT/ΔC mice, PASD mice, and all wild-type mice demonstrated typical behavioral responses in most tests.
Limitations: This study tested the interaction between developmental sleep disruption and genetic vulnerability using a single ASD mouse model: Shank3ΔC (deletion of exon 21). The broader implications of this work should be supported by additional studies using ASD model mice with distinct genetic vulnerabilities.
Conclusion: Our study shows that sleep disruption during sensitive periods of early life interacts with underlying genetic vulnerability to drive lasting and sex-specific changes in behavior. As individuals progress through maturation, they gain resilience to the lasting effects of sleep disruption. This work highlights developmental sleep disruption as an important vulnerability in ASD susceptibility.
Keywords: Autism spectrum disorder; Brain development; Shank3; Sleep; Social behavior.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Major motor and gait deficits with sexual dimorphism in a Shank3 mutant mouse model.Mol Autism. 2021 Jan 19;12(1):2. doi: 10.1186/s13229-020-00412-8. Mol Autism. 2021. PMID: 33468258 Free PMC article.
-
Absence of familiarity triggers hallmarks of autism in mouse model through aberrant tail-of-striatum and prelimbic cortex signaling.Neuron. 2022 May 4;110(9):1468-1482.e5. doi: 10.1016/j.neuron.2022.02.001. Epub 2022 Feb 25. Neuron. 2022. PMID: 35219402
-
Sensitivity to isoflurane anesthesia increases in autism spectrum disorder Shank3+/∆c mutant mouse model.Neurotoxicol Teratol. 2017 Mar-Apr;60:69-74. doi: 10.1016/j.ntt.2016.11.002. Epub 2016 Nov 14. Neurotoxicol Teratol. 2017. PMID: 27856360 Free PMC article.
-
Comparison of SHANK3 deficiency in animal models: phenotypes, treatment strategies, and translational implications.J Neurodev Disord. 2021 Nov 16;13(1):55. doi: 10.1186/s11689-021-09397-8. J Neurodev Disord. 2021. PMID: 34784886 Free PMC article. Review.
-
SHANK3 variant as a cause of nonsyndromal autism in an 11-year-old boy and a review of published literature.Clin Dysmorphol. 2018 Oct;27(4):113-115. doi: 10.1097/MCD.0000000000000232. Clin Dysmorphol. 2018. PMID: 29939863 Review.
Cited by
-
Critical periods and Autism Spectrum Disorders, a role for sleep.Neurobiol Sleep Circadian Rhythms. 2022 Dec 20;14:100088. doi: 10.1016/j.nbscr.2022.100088. eCollection 2023 May. Neurobiol Sleep Circadian Rhythms. 2022. PMID: 36632570 Free PMC article.
-
Adolescent sleep and its disruption in depression and anxiety.Front Neurosci. 2024 Nov 7;18:1479420. doi: 10.3389/fnins.2024.1479420. eCollection 2024. Front Neurosci. 2024. PMID: 39575099 Free PMC article. Review.
-
Developing forebrain synapses are uniquely vulnerable to sleep loss.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2407533121. doi: 10.1073/pnas.2407533121. Epub 2024 Oct 23. Proc Natl Acad Sci U S A. 2024. PMID: 39441640
-
Analysis of hepatic lentiviral vector transduction; implications for preclinical studies and clinical gene therapy protocols.bioRxiv [Preprint]. 2024 Aug 31:2024.08.20.608805. doi: 10.1101/2024.08.20.608805. bioRxiv. 2024. PMID: 39229157 Free PMC article. Preprint.
-
Sex-Specific Impacts of Early Life Sleep Disruption: Ethanol Seeking, Social Interaction, and Anxiety Are Differentially Altered in Adolescent Prairie Voles.Dev Psychobiol. 2024 Nov;66(7):e22541. doi: 10.1002/dev.22541. Dev Psychobiol. 2024. PMID: 39192630
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

