Negative Feedback of the cAMP/PKA Pathway Regulates the Effects of Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome Activation on Type II Alveolar Epithelial Cell Pyroptosis as a Novel Mechanism of BLM-Induced Pulmonary Fibrosis
- PMID: 36033388
- PMCID: PMC9410862
- DOI: 10.1155/2022/2291877
Negative Feedback of the cAMP/PKA Pathway Regulates the Effects of Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome Activation on Type II Alveolar Epithelial Cell Pyroptosis as a Novel Mechanism of BLM-Induced Pulmonary Fibrosis
Abstract
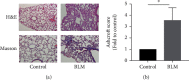
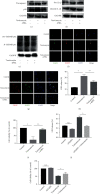
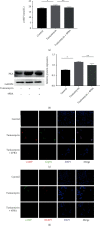
Endoplasmic reticulum stress (ER stress) contributes to the development of pulmonary fibrosis, especially in type II alveolar epithelial cells (AECs) apoptosis. ER stress also promotes NLRP3 inflammasome activation which is inhibited by upregulation of cAMP/PKA pathway. However, it is confused whether ER stress-induced NLRP3 inflammasome activation and pyroptosis in type II alveolar epithelial cells which exacerbates pulmonary fibrosis via a mechanism that is suppressed by cAMP/PKA pathway. In our research, we explored that potential links among NLRP3 inflammasome, ER stress, and cAMP/PKA pathway in type II AECs to explain the new mechanisms of pulmonary fibrosis. We found that in vivo, ER stress, NLRP3 inflammasome, and PKA upregulated in the alveolar epithelial area in animal models of pulmonary fibrosis. In addition, immunofluorescence staining further confirmed that ER stress, NLRP3 inflammasome, and cAMP/PKA had potential links on type II AECs in BLM group. In vitro, ER stress stimulated NLRP3 inflammasome activation, promoted pyroptosis, and also upregulated cAMP/PKA pathway. Upregulation of cAMP/PKA pathway inhibited ER stress-induced pyroptosis of A549 cells and vice versa. These results initially supported conclusion that ER stress may stimulate NLRP3 inflammasome activation and pyroptosis in type II AECs, which exacerbated pulmonary fibrosis, and cAMP/PKA pathway may act as a feedback regulator.
Copyright © 2022 Qiaohui Hong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Bone morphogenetic protein 4 ameliorates bleomycin-induced pulmonary fibrosis in mice by repressing NLRP3 inflammasome activation and epithelial-mesenchymal transition.J Thorac Dis. 2024 Aug 31;16(8):4875-4891. doi: 10.21037/jtd-23-1947. Epub 2024 Aug 28. J Thorac Dis. 2024. PMID: 39268124 Free PMC article.
-
NLRP3 inflammasome activation in alveolar epithelial cells promotes myofibroblast differentiation of lung-resident mesenchymal stem cells during pulmonary fibrogenesis.Biochim Biophys Acta Mol Basis Dis. 2021 May 1;1867(5):166077. doi: 10.1016/j.bbadis.2021.166077. Epub 2021 Jan 27. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33515677
-
Toll-Like Receptor 9 Aggravates Pulmonary Fibrosis by Promoting NLRP3-Mediated Pyroptosis of Alveolar Epithelial Cells.Inflammation. 2024 Oct;47(5):1744-1761. doi: 10.1007/s10753-024-02006-5. Epub 2024 Mar 18. Inflammation. 2024. PMID: 38498270
-
The Roles of Endoplasmic Reticulum in NLRP3 Inflammasome Activation.Cells. 2020 May 14;9(5):1219. doi: 10.3390/cells9051219. Cells. 2020. PMID: 32423023 Free PMC article. Review.
-
ER Stress Activates the NLRP3 Inflammasome: A Novel Mechanism of Atherosclerosis.Oxid Med Cell Longev. 2019 Oct 7;2019:3462530. doi: 10.1155/2019/3462530. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31687078 Free PMC article. Review.
Cited by
-
Perspectives of PDE inhibitor on treating idiopathic pulmonary fibrosis.Front Pharmacol. 2023 Feb 14;14:1111393. doi: 10.3389/fphar.2023.1111393. eCollection 2023. Front Pharmacol. 2023. PMID: 36865908 Free PMC article. Review.
-
Astragaloside IV alleviates renal fibrosis by inhibiting renal tubular epithelial cell pyroptosis induced by urotensin II through regulating the cAMP/PKA signaling pathway.PLoS One. 2024 May 31;19(5):e0304365. doi: 10.1371/journal.pone.0304365. eCollection 2024. PLoS One. 2024. PMID: 38820434 Free PMC article.
-
LncRNA-mediated ceRNA network reveals the mechanism of action of Saorilao-4 decoction against pulmonary fibrosis.Front Genet. 2024 Mar 12;15:1339064. doi: 10.3389/fgene.2024.1339064. eCollection 2024. Front Genet. 2024. PMID: 38533208 Free PMC article.
-
Ficolin B secreted by alveolar macrophage exosomes exacerbates bleomycin-induced lung injury via ferroptosis through the cGAS-STING signaling pathway.Cell Death Dis. 2023 Aug 30;14(8):577. doi: 10.1038/s41419-023-06104-4. Cell Death Dis. 2023. PMID: 37648705 Free PMC article.
-
Regulation of alveolar macrophage death in pulmonary fibrosis: a review.Apoptosis. 2023 Dec;28(11-12):1505-1519. doi: 10.1007/s10495-023-01888-4. Epub 2023 Sep 14. Apoptosis. 2023. PMID: 37707713 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

