Nature vs. nurture: FOXP3, genetics, and tissue environment shape Treg function
- PMID: 36032083
- PMCID: PMC9411801
- DOI: 10.3389/fimmu.2022.911151
Nature vs. nurture: FOXP3, genetics, and tissue environment shape Treg function
Abstract
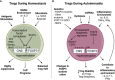
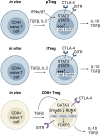
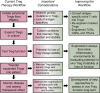
The importance of regulatory T cells (Tregs) in preventing autoimmunity has been well established; however, the precise alterations in Treg function in autoimmune individuals and how underlying genetic associations impact the development and function of Tregs is still not well understood. Polygenetic susceptibly is a key driving factor in the development of autoimmunity, and many of the pathways implicated in genetic association studies point to a potential alteration or defect in regulatory T cell function. In this review transcriptomic control of Treg development and function is highlighted with a focus on how these pathways are altered during autoimmunity. In combination, observations from autoimmune mouse models and human patients now provide insights into epigenetic control of Treg function and stability. How tissue microenvironment influences Treg function, lineage stability, and functional plasticity is also explored. In conclusion, the current efficacy and future direction of Treg-based therapies for Type 1 Diabetes and other autoimmune diseases is discussed. In total, this review examines Treg function with focuses on genetic, epigenetic, and environmental mechanisms and how Treg functions are altered within the context of autoimmunity.
Keywords: FOXP3; T cell; Treg - regulatory T cell; autoimmunity; genetic; type 1 diabetes.
Copyright © 2022 Raugh, Allard and Bettini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases.Clin Exp Immunol. 2019 Jul;197(1):24-35. doi: 10.1111/cei.13288. Epub 2019 Mar 24. Clin Exp Immunol. 2019. PMID: 30830965 Free PMC article. Review.
-
Few Foxp3⁺ regulatory T cells are sufficient to protect adult mice from lethal autoimmunity.Eur J Immunol. 2014 Oct;44(10):2990-3002. doi: 10.1002/eji.201344315. Epub 2014 Aug 11. Eur J Immunol. 2014. PMID: 25042334
-
Regulatory T cell metabolism at the intersection between autoimmune diseases and cancer.Eur J Immunol. 2020 Nov;50(11):1626-1642. doi: 10.1002/eji.201948470. Epub 2020 Oct 26. Eur J Immunol. 2020. PMID: 33067808 Free PMC article. Review.
-
Mechanisms of human FoxP3+ Treg cell development and function in health and disease.Clin Exp Immunol. 2019 Jul;197(1):36-51. doi: 10.1111/cei.13290. Epub 2019 Apr 1. Clin Exp Immunol. 2019. PMID: 30864147 Free PMC article. Review.
-
Transient Depletion of Foxp3+ Regulatory T Cells Selectively Promotes Aggressive β Cell Autoimmunity in Genetically Susceptible DEREG Mice.Front Immunol. 2021 Aug 10;12:720133. doi: 10.3389/fimmu.2021.720133. eCollection 2021. Front Immunol. 2021. PMID: 34447385 Free PMC article.
Cited by
-
Treatment with a Lactococcus lactis that chromosomally express E. coli cfaI mitigates salivary flow loss in a Sjögren's syndrome-like disease.Sci Rep. 2023 Nov 9;13(1):19489. doi: 10.1038/s41598-023-46557-3. Sci Rep. 2023. PMID: 37945636 Free PMC article.
-
T-lymphoid progenitor-based immunotherapies: clinical perspectives for one and all.Cell Mol Immunol. 2022 Dec;19(12):1435-1438. doi: 10.1038/s41423-022-00927-5. Epub 2022 Sep 30. Cell Mol Immunol. 2022. PMID: 36180781 Free PMC article. No abstract available.
-
Investigating the Role of FoxP3 in Renal Cell Carcinoma Metastasis with BAP1 or SEDT2 Mutation.Int J Mol Sci. 2023 Aug 1;24(15):12301. doi: 10.3390/ijms241512301. Int J Mol Sci. 2023. PMID: 37569676 Free PMC article.
-
Gut microbiome, metabolome and alopecia areata.Front Microbiol. 2023 Nov 15;14:1281660. doi: 10.3389/fmicb.2023.1281660. eCollection 2023. Front Microbiol. 2023. PMID: 38033589 Free PMC article. Review.
-
The impact of FOXP3 polymorphisms on oral cancer progression and clinicopathological characteristics.J Cancer. 2023 May 5;14(7):1195-1201. doi: 10.7150/jca.84470. eCollection 2023. J Cancer. 2023. PMID: 37215447 Free PMC article.
References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol Baltim Md 1950 (1995) 155:1151–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

