A Prospective Observational Study on BBV152 Coronavirus Vaccine Use in Adolescents and Comparison with Adults: Interim Results of the First Real-World Safety Analysis
- PMID: 36030299
- PMCID: PMC9419918
- DOI: 10.1007/s40264-022-01226-8
A Prospective Observational Study on BBV152 Coronavirus Vaccine Use in Adolescents and Comparison with Adults: Interim Results of the First Real-World Safety Analysis
Abstract
Introduction: The BBV152 coronavirus disease 2019 (COVID-19) vaccine (COVAXIN) has recently been approved for adolescents.
Objective: We provide the first real-world safety data of COVAXIN use in adolescents and compare with adults.
Methods: A prospective observational study was initiated in January 2022. Enrolled adolescents and adults were contacted by telephone after 14 days of receiving the BBV152 vaccine. The primary outcome was vaccine safety assessed as rate of adverse events following immunization (AEFIs). Severity grading of AEFIs was done using the Food and Drug Administration (FDA) scale. Interim results are presented.
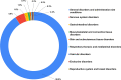
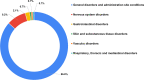
Results: A total of 698 adolescents and 326 adults were enrolled. AEFIs after the first dose developed in 243 out of 670 adolescents (36.3%), with 21% reporting only local AEFIs and 15.2% reporting systemic AEFIs. Among 340 adolescents who had received the second dose of vaccine, 129 (37.9%) developed AEFIs, with only local involvement in 20.3% and systemic involvement in 17.6%. Injection site pain and fever were the common AEFIs. The majority of AEFIs were mild-moderate. Nearly 0.9% of adolescents receiving the first dose reported severe AEFIs. Atypical AEFIs were observed in 0.6-0.9% of adolescents. The majority of the AEFIs resolved in 1-2 days. AEFIs were persistent in > 2% of adolescents at day 14 after the second dose, and also in 3.7% of adults overall at follow-up. No difference was observed in AEFI incidence and patterns between adolescents and adults. Regression analysis showed females and those with a history of allergy to be, respectively, at 1.6 times and 3 times increased risk of AEFIs among adolescents.
Conclusions: COVAXIN carries an overall favorable short-term safety profile in adolescents. The observed AEFI rates in adolescents are much lower than that reported with mRNA vaccines, but head-head comparisons in the same population are required to generate relative vaccine safety data. Female adolescents and those with a history of allergy need watchfulness for severe and persistent AEFIs. With some AEFIs persisting at 14 days, a longer follow-up is recommended to strengthen the safety data of COVAXIN.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands.Drug Saf. 2022 Apr;45(4):319-331. doi: 10.1007/s40264-022-01151-w. Epub 2022 Mar 21. Drug Saf. 2022. PMID: 35314943 Free PMC article.
-
Active Safety Surveillance of Four Types of COVID-19 Vaccines: A National Study from Jordan.Clin Drug Investig. 2022 Oct;42(10):813-827. doi: 10.1007/s40261-022-01191-1. Epub 2022 Aug 23. Clin Drug Investig. 2022. PMID: 35999428 Free PMC article.
-
COVID-19 mRNA Vaccines Are Generally Safe in the Short Term: A Vaccine Vigilance Real-World Study Says.Front Immunol. 2021 May 21;12:669010. doi: 10.3389/fimmu.2021.669010. eCollection 2021. Front Immunol. 2021. PMID: 34093567 Free PMC article.
-
Sex-disaggregated outcomes of adverse events after COVID-19 vaccination: A Dutch cohort study and review of the literature.Front Immunol. 2023 Jan 30;14:1078736. doi: 10.3389/fimmu.2023.1078736. eCollection 2023. Front Immunol. 2023. PMID: 36793715 Free PMC article. Review.
-
Adverse events following immunization: real causality and myths.Expert Opin Drug Saf. 2016 Jun;15(6):825-35. doi: 10.1517/14740338.2016.1167869. Epub 2016 Apr 1. Expert Opin Drug Saf. 2016. PMID: 26986067 Review.
Cited by
-
Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis.Front Public Health. 2024 Jul 30;12:1406315. doi: 10.3389/fpubh.2024.1406315. eCollection 2024. Front Public Health. 2024. PMID: 39139673 Free PMC article.
-
RETRACTED ARTICLE: Long-Term Safety Analysis of the BBV152 Coronavirus Vaccine in Adolescents and Adults: Findings from a 1-Year Prospective Study in North India.Drug Saf. 2024 May 13. doi: 10.1007/s40264-024-01432-6. Online ahead of print. Drug Saf. 2024. PMID: 38740691 No abstract available.
-
COVID-19 Vaccine Uptake in Immigrant, Refugee, and Nonimmigrant Children and Adolescents in Ontario, Canada.JAMA Netw Open. 2023 Jul 3;6(7):e2325636. doi: 10.1001/jamanetworkopen.2023.25636. JAMA Netw Open. 2023. PMID: 37494039 Free PMC article.
-
Application of Traditional Vaccine Development Strategies to SARS-CoV-2.mSystems. 2023 Apr 27;8(2):e0092722. doi: 10.1128/msystems.00927-22. Epub 2023 Mar 2. mSystems. 2023. PMID: 36861991 Free PMC article. Review.
-
Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2-18 years: an updated systematic review and meta-analysis.World J Pediatr. 2023 Nov;19(11):1041-1054. doi: 10.1007/s12519-022-00680-9. Epub 2023 Feb 1. World J Pediatr. 2023. PMID: 36723827 Free PMC article. Review.
References
-
- World Health Organization. Interim statement on COVID-19 vaccination for children and adolescents. https://www.who.int/news/item/24-11-2021-interim-statement-on-covid-19-v....
-
- US FDA. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic [Internet]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... [cited 2022 Apr 7].
-
- China.org.cn. Sinovac COVID-19 vaccine safe for children, adolescents [Internet]. http://www.china.org.cn/china/2021-07/02/content_77602836.htm [cited 2022 Apr 7].
-
- Vadrevu KM, Reddy S, Jogdand H, Ganneru B, Mirza N, Tripathy VN, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (BBV152) in children from 2 to 18 years of age: an open-label, age-de-escalation phase 2/3 study. medRxiv [Internet]. 2021;2021.12.28.21268468. http://medrxiv.org/content/early/2021/12/29/2021.12.28.21268468.abstract. - PubMed
-
- Kaur U, Anju KL, Chauhan M, Joshi A, Kansal S, Jaisawal V, et al. A prospective observational study on BBV152 coronavirus vaccine use in adolescents and comparison with adults- first real-world safety analysis. medRxiv [Internet]. 2022;2022.04.08.22273634. http://medrxiv.org/content/early/2022/04/10/2022.04.08.22273634.abstract. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

