Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c+ dendritic cells
- PMID: 36030251
- PMCID: PMC9420140
- DOI: 10.1038/s41419-022-05191-z
Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c+ dendritic cells
Abstract
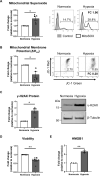
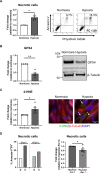
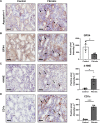
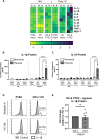
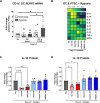
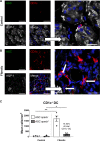
Inflammasomes are multiprotein platforms responsible for the release of pro-inflammatory cytokines interleukin (IL)-1β and IL-18. Mouse studies have identified inflammasome activation within dendritic cells (DC) as pivotal for driving tubulointerstitial fibrosis and inflammation, the hallmarks of chronic kidney disease (CKD). However, translation of this work to human CKD remains limited. Here, we examined the complex tubular cell death pathways mediating inflammasome activation in human kidney DC and, thus, CKD progression. Ex vivo patient-derived proximal tubular epithelial cells (PTEC) cultured under hypoxic (1% O2) conditions modelling the CKD microenvironment showed characteristics of ferroptotic cell death, including mitochondrial dysfunction, reductions in the lipid repair enzyme glutathione peroxidase 4 (GPX4) and increases in lipid peroxidation by-product 4-hydroxynonenal (4-HNE) compared with normoxic PTEC. The addition of ferroptosis inhibitor, ferrostatin-1, significantly reduced hypoxic PTEC death. Human CD1c+ DC activated in the presence of hypoxic PTEC displayed significantly increased production of inflammasome-dependent cytokines IL-1β and IL-18. Treatment of co-cultures with VX-765 (caspase-1/4 inhibitor) and MCC950 (NLRP3 inflammasome inhibitor) significantly attenuated IL-1β/IL-18 levels, supporting an NLRP3 inflammasome-dependent DC response. In line with these in vitro findings, in situ immunolabelling of human fibrotic kidney tissue revealed a significant accumulation of tubulointerstitial CD1c+ DC containing active inflammasome (ASC) specks adjacent to ferroptotic PTEC. These data establish ferroptosis as the primary pattern of PTEC necrosis under the hypoxic conditions of CKD. Moreover, this study identifies NLRP3 inflammasome signalling driven by complex tubulointerstitial PTEC-DC interactions as a key checkpoint for therapeutic targeting in human CKD.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Human proximal tubular epithelial cell-derived small extracellular vesicles mediate synchronized tubular ferroptosis in hypoxic kidney injury.Redox Biol. 2024 Apr;70:103042. doi: 10.1016/j.redox.2024.103042. Epub 2024 Jan 14. Redox Biol. 2024. PMID: 38244399 Free PMC article.
-
Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases.Kidney Int. 2019 Jul;96(1):58-66. doi: 10.1016/j.kint.2019.01.014. Epub 2019 Mar 4. Kidney Int. 2019. PMID: 30922667 Review.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury.Oncotarget. 2016 Apr 5;7(14):17479-91. doi: 10.18632/oncotarget.8243. Oncotarget. 2016. PMID: 27014913 Free PMC article.
-
The IL-18/IL-18R1 signalling axis: Diagnostic and therapeutic potential in hypertension and chronic kidney disease.Pharmacol Ther. 2022 Nov;239:108191. doi: 10.1016/j.pharmthera.2022.108191. Epub 2022 Apr 21. Pharmacol Ther. 2022. PMID: 35461924 Review.
Cited by
-
Lupus Nephritis: Immune Cells and the Kidney Microenvironment.Kidney360. 2024 Sep 1;5(9):1394-1401. doi: 10.34067/KID.0000000000000531. Epub 2024 Aug 9. Kidney360. 2024. PMID: 39120952 Free PMC article. Review.
-
The immunoregulatory roles of non-haematopoietic cells in the kidney.Nat Rev Nephrol. 2024 Apr;20(4):206-217. doi: 10.1038/s41581-023-00786-x. Epub 2023 Nov 20. Nat Rev Nephrol. 2024. PMID: 37985868 Free PMC article. Review.
-
Regulated cell death in chronic kidney disease: current evidence and future clinical perspectives.Front Cell Dev Biol. 2024 Oct 31;12:1497460. doi: 10.3389/fcell.2024.1497460. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39544363 Free PMC article. Review.
-
The Road from AKI to CKD: Molecular Mechanisms and Therapeutic Targets of Ferroptosis.Cell Death Dis. 2023 Jul 13;14(7):426. doi: 10.1038/s41419-023-05969-9. Cell Death Dis. 2023. PMID: 37443140 Free PMC article. Review.
-
Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

