Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antigen detection in the Emergency Department: data from a pediatric cohort during the fourth COVID-19 wave in Italy
- PMID: 36028877
- PMCID: PMC9412777
- DOI: 10.1186/s13052-022-01343-1
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antigen detection in the Emergency Department: data from a pediatric cohort during the fourth COVID-19 wave in Italy
Abstract
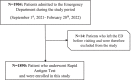
Background: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic has been challenging health care systems and made it necessary to use rapid and cost-effective testing methods, particularly in Emergency Department (ED) settings. Rapid Antigen Diagnostic Tests (RADTs) are a valid alternative to the gold standard RT-PCR, even in pediatric populations. This retrospective observational study has been conducted on a pediatric cohort afferent to the ED of the San Giovanni di Dio and Ruggi d'Aragona University Hospital in Salerno, tested at Point of Care with RADT Panbio® (Abbott), from September 1st, 2021 to February 28th, 2022, analyzing the positivity rate and clinical features of the cohort, also in reference to the rise of positive cases observed in the aforementioned period, and to the introduction in Italy of SARS-CoV-2 vaccination for children and teens on December 16th, 2021.
Methods: Data regarding access to the pediatric ED were extracted from the hospital's electronic database system. Parallel to this, we conducted a narrative literature search using PubMed database focusing on the use of RADT in pediatric ED and compared our data with the national pandemic trend.
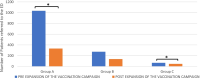
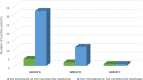
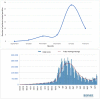
Results: During the observation period, 1890 patients were tested for the presence of SARS-CoV-2 with RADT and the 2.7% of children resulted positive, with a peak in January 2022. The main symptoms in positive patients were: fever (n = 34; 66.7%), cough (n = 11; 21.5%), headache (n = 4; 7.8%), chest pain (n = 2; 3.9%) and abdominal pain (n = 1; 2%). Patients were divided into three different age groups (A, B, C) basing on the different access timing to vaccination; no statistically significant difference was detected in the distribution of positivity in these three groups (p > 0.05). Number of positive children in group A was greater in the post-vaccine group. Our data are concordant with the national trend of the pandemic showing a fourth wave peak in January 2022.
Conclusion: The use of RADT as a first point-of-care screening may be helpful, time-saving and cost-sparing. Our study shows that, during the observation period, most children admitted to the ED for fever, actually tested positive for SARS-CoV-2 with a statistically greater difference than negative children. Instead, number of patients admitted for cough was statistically higher among negative than positive ones, probably due to the circulation of other respiratory viruses in children.
Keywords: Children; Emergency department; Rapid antigen diagnostic tests (RADTs); SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Evaluation of the Rapid Antigen Detection Test for Diagnosing SARS-CoV-2 during the COVID-19 Pandemic: Experience from a Centralized Isolation Site in Shanghai, China.Microbiol Spectr. 2023 Feb 14;11(1):e0454222. doi: 10.1128/spectrum.04542-22. Epub 2023 Jan 19. Microbiol Spectr. 2023. PMID: 36655994 Free PMC article.
-
A cross-sectional study of screening for coronavirus disease 2019 (COVID-19) at the pediatric emergency department in Vilnius during the first wave of the pandemic.Eur J Pediatr. 2021 Jul;180(7):2137-2145. doi: 10.1007/s00431-021-03999-z. Epub 2021 Feb 25. Eur J Pediatr. 2021. PMID: 33634336 Free PMC article.
-
[Evaluation of SARS-CoV-2 PCR Positive Cases in the Pediatric Emergency Department].Mikrobiyol Bul. 2020 Oct;54(4):629-637. doi: 10.5578/mb.70086. Mikrobiyol Bul. 2020. PMID: 33107292 Turkish.
-
Diagnosis of COVID-19 in children guided by lack of fever and exposure to SARS-CoV-2.Pediatr Res. 2022 Apr;91(5):1196-1202. doi: 10.1038/s41390-021-01585-5. Epub 2021 Jun 11. Pediatr Res. 2022. PMID: 34117360 Free PMC article. Review.
-
Human and novel coronavirus infections in children: a review.Paediatr Int Child Health. 2021 Feb;41(1):36-55. doi: 10.1080/20469047.2020.1781356. Epub 2020 Jun 25. Paediatr Int Child Health. 2021. PMID: 32584199 Review.
References
-
- Mauro A, Improda N, Zenzeri L, Valitutti F, Vecchione E, Esposito S, et al. Infection control strategy and primary care assistance in Campania region during the national lockdown due to COVID-19 outbreak: the experience of two tertiary emergency centers. Ital J Pediatr. 2021;47:19. doi: 10.1186/s13052-021-00963-3. - DOI - PMC - PubMed
-
- Carbonell-Sahuquillo S, Lázaro-Carreño MI, Camacho J, Barrés-Fernández A, Albert E, Torres I, et al. Evaluation of a rapid antigen detection test (Panbio™ COVID-19 ag rapid test device) as a point-of-care diagnostic tool for COVID-19 in a pediatric emergency department. J Med Virol. 2021;93:6803–6807. doi: 10.1002/jmv.27220. - DOI - PMC - PubMed
-
- Villaverde S, Domínguez-Rodríguez S, Sabrido G, Pérez-Jorge C, Plata M, Romero MP, et al. Diagnostic accuracy of the Panbio severe acute respiratory syndrome coronavirus 2 antigen rapid test compared with reverse-transcriptase polymerase chain reaction testing of nasopharyngeal samples in the pediatric population. J Pediatr. 2021;232:287–289. doi: 10.1016/j.jpeds.2021.01.027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

