Sclerostin is involved in osteogenic transdifferentiation of vascular smooth muscle cells in chronic kidney disease-associated vascular calcification with non-canonical Wnt signaling
- PMID: 36017689
- PMCID: PMC9423850
- DOI: 10.1080/0886022X.2022.2114370
Sclerostin is involved in osteogenic transdifferentiation of vascular smooth muscle cells in chronic kidney disease-associated vascular calcification with non-canonical Wnt signaling
Abstract
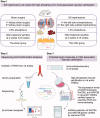
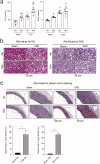
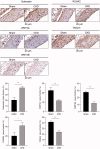
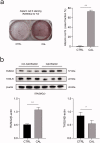
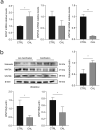
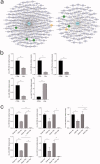
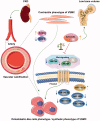
Vascular calcification is prominent in patients with chronic kidney disease (CKD) and is a strong predictor of cardiovascular mortality in the CKD population. However, the mechanism underlying CKD-associated vascular calcification remains unclear. To identify potential therapeutic targets, a 5/6 nephrectomy rat model was established by feeding of a high-phosphorous diet as the CKD group and compared with sham group rats at 4 and 16 weeks. Sequencing analyses of the rat aorta revealed 643 upregulated and 1023 downregulated genes at 4 weeks, as well as 899 upregulated and 1185 downregulated genes at 16 weeks in the CKD group compared to the sham group. Bioinformatics analyses suggested that SOST (which encodes sclerostin) and Wnt signaling are involved in CKD-associated vascular calcification. Furthermore, protein-protein interactions analysis revealed interactions between SOST, WNT5A, and WNT5B, that involved runt-related transcription factor 2 (RUNX2) and transgelin (TAGLN). SOST was increased in CKD-associated vascular calcification following reduction of the Wnt signaling, including WNT5A and WNT5B, both in vivo and in vitro. TargetScan was used to predict the microRNAs (miRNAs) targeting WNT5A and WNT5B. The expression levels of miR-542-3p, miR-298-3p, miR-376b-5p, and miR-3568 were significantly reduced, whereas that of miR-742-3p was significantly increased in calcified rat aortic vascular smooth muscle cells (VSMCs). In CKD rat aortas, the expression of miR-542-3p, miR-298-3p, miR-376b-5p, miR-3568, miR-742-3p, and miR-22-5p were significantly reduced at both 4 and 16 weeks. Altogether, owing to several assessments, potentially diagnostic and prognostic biomarkers for improving common CKD diagnostic tools were identified in this study. Abbreviations: BUN: blood urea nitrogen; CKD: chronic kidney disease; CKD-MBD: chronic kidney disease-mineral bone disorder; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GO: the Gene Ontology; HE: hematoxylin-eosin; HRP: horseradish peroxidase; KEGG: Kyoto Encyclopedia of Genes and Genomes; MiRNAs: microRNAs; PAS: periodic acid-Schiff; RUNX2: runt-related transcription factor 2; SCr: serum creatinine; STRING: the Search Tool for the Retrieval of Interacting Genes/Proteins; TAGLN: transgelin; VSMC: vascular smooth muscle cell.
Keywords: Chronic kidney disease; Wnt signaling pathway; microRNA; sclerostin; vascular calcification; vascular smooth muscle cells.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Circulating Extracellular Vesicle-Propagated microRNA Signature as a Vascular Calcification Factor in Chronic Kidney Disease.Circ Res. 2023 Feb 17;132(4):415-431. doi: 10.1161/CIRCRESAHA.122.321939. Epub 2023 Jan 26. Circ Res. 2023. PMID: 36700539
-
High phosphorus level leads to aortic calcification via β-catenin in chronic kidney disease.Am J Nephrol. 2015;41(1):28-36. doi: 10.1159/000370250. Epub 2015 Jan 24. Am J Nephrol. 2015. PMID: 25634106
-
MicroRNAs 29b, 133b, and 211 Regulate Vascular Smooth Muscle Calcification Mediated by High Phosphorus.J Am Soc Nephrol. 2016 Mar;27(3):824-34. doi: 10.1681/ASN.2014050520. Epub 2015 Jul 17. J Am Soc Nephrol. 2016. PMID: 26187577 Free PMC article.
-
Key regulators of vascular calcification in chronic kidney disease: Hyperphosphatemia, BMP2, and RUNX2.PeerJ. 2024 Sep 19;12:e18063. doi: 10.7717/peerj.18063. eCollection 2024. PeerJ. 2024. PMID: 39308809 Free PMC article. Review.
-
Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia.Cell Mol Life Sci. 2019 Jun;76(11):2077-2091. doi: 10.1007/s00018-019-03054-z. Epub 2019 Mar 18. Cell Mol Life Sci. 2019. PMID: 30887097 Free PMC article. Review.
Cited by
-
Integrin-linked kinase mRNA expression in circulating mononuclear cells as a biomarker of kidney and vascular damage in experimental chronic kidney disease.Cell Commun Signal. 2024 May 11;22(1):264. doi: 10.1186/s12964-024-01646-2. Cell Commun Signal. 2024. PMID: 38734696 Free PMC article.
References
-
- Zhang L, Wang F, Wang L, et al. . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. - PubMed
-
- Liu ZH, Yu XQ, Yang JW, et al. . Prevalence and risk factors for vascular calcification in Chinese patients receiving dialysis: baseline results from a prospective cohort study. Curr Med Res Opin. 2018;34(8):1491–1500. - PubMed
-
- Vervloet M, Cozzolino M.. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 2017;91(4):808–817. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
