LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression
- PMID: 36016134
- PMCID: PMC9414238
- DOI: 10.3390/vaccines10081246
LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression
Abstract
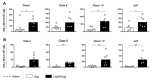
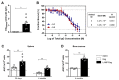
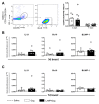
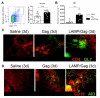
Neonates have a limited adaptive response of plasma cells, germinal center (GC) B cells, and T follicular helper cells (TFH). As neonatal vaccination can be an important tool for AIDS prevention, these limitations need to be overcome. Chimeric DNA vaccine encoding p55Gag HIV-1 protein conjugated with lysosomal-associated membrane protein 1 (LAMP-1) has been described as immunogenic in the neonate period. Herein, we investigated the immunologic mechanisms involved in neonatal immunization with a LAMP-1/p55Gag (LAMP/Gag) DNA vaccine in a C57BL/6 mouse background. Neonatal LAMP/Gag vaccination induced strong Gag-specific T-cell response until adulthood and elevated levels of anti-Gag IgG antibodies. We also demonstrated for the first time that the immunogenicity of the neonatal period with LAMP/Gag is due to the induction of high-affinity anti-p24 IgG antibodies and long-term plasma cells. Together with that, there is the generation of early TFH cells and the formation of GC sites with the upregulation of activation-induced cytidine deaminase (AID) enzyme mRNA and protein expression in draining lymph nodes after neonatal LAMP/Gag vaccination. These findings underscore that the LAMP-1 strategy in the chimeric vaccine could be useful to enhance antibody production even in the face of neonatal immaturity, and they contribute to the development of new vaccine approaches for other emerging pathogens at an early stage of life.
Keywords: DNA vaccine; HIV; germinal center; immunogenicity; neonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mucosal and systemic anti-GAG immunity induced by neonatal immunization with HIV LAMP/gag DNA vaccine in mice.Immunobiology. 2011 Apr;216(4):505-12. doi: 10.1016/j.imbio.2010.08.007. Epub 2010 Sep 25. Immunobiology. 2011. PMID: 20870310
-
Immunization of neonatal mice with LAMP/p55 HIV gag DNA elicits robust immune responses that last to adulthood.Virology. 2010 Oct 10;406(1):37-47. doi: 10.1016/j.virol.2010.06.050. Epub 2010 Jul 29. Virology. 2010. PMID: 20667577
-
Maternal LAMP/p55gagHIV-1 DNA immunization induces in utero priming and a long-lasting immune response in vaccinated neonates.PLoS One. 2012;7(2):e31608. doi: 10.1371/journal.pone.0031608. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355381 Free PMC article.
-
Harnessing T Follicular Helper Cell Responses for HIV Vaccine Development.Viruses. 2018 Jun 19;10(6):336. doi: 10.3390/v10060336. Viruses. 2018. PMID: 29921828 Free PMC article. Review.
-
Tfh1 Cells in Germinal Centers During Chronic HIV/SIV Infection.Front Immunol. 2018 Jun 6;9:1272. doi: 10.3389/fimmu.2018.01272. eCollection 2018. Front Immunol. 2018. PMID: 29928280 Free PMC article. Review.
Cited by
-
Lysosome-Associated Membrane Protein Targeting Strategy Improved Immunogenicity of Glycoprotein-Based DNA Vaccine for Marburg Virus.Vaccines (Basel). 2024 Sep 4;12(9):1013. doi: 10.3390/vaccines12091013. Vaccines (Basel). 2024. PMID: 39340043 Free PMC article.
-
Enhanced immunogenicity and protective efficacy in mice following a Zika DNA vaccine designed by modulation of membrane-anchoring regions and its association to adjuvants.Front Immunol. 2024 Jan 19;15:1307546. doi: 10.3389/fimmu.2024.1307546. eCollection 2024. Front Immunol. 2024. PMID: 38361945 Free PMC article.
References
-
- UNAIDS. Global HIV&AIDS Statistics—Fact Sheet 2022. [(accessed on 30 July 2022)]. Available online: https://www.unaids.org/en/resources/fact-sheet#:~:text=38.4%20million%20....
-
- Pihlgren M., Tougne C., Bozzotti P., Fulurija A., Duchosal M.A., Lambert P.H., Siegrist C.A. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 2003;170:2824–2832. doi: 10.4049/jimmunol.170.6.2824. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

