Conformational Changes of α-Crystallin Proteins Induced by Heat Stress
- PMID: 36012609
- PMCID: PMC9409278
- DOI: 10.3390/ijms23169347
Conformational Changes of α-Crystallin Proteins Induced by Heat Stress
Abstract
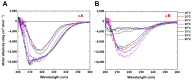
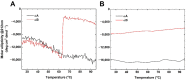
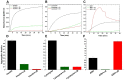
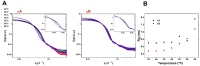
α-crystallin is a major structural protein in the eye lenses of vertebrates that is composed of two relative subunits, αA and αB crystallin, which function in maintaining lens transparency. As a member of the small heat-shock protein family (sHsp), α-crystallin exhibits chaperone-like activity to prevent the misfolding or aggregation of critical proteins in the lens, which is associated with cataract disease. In this study, high-purity αA and αB crystallin proteins were expressed from E. coli and purified by affinity and size-exclusion chromatography. The size-exclusion chromatography experiment showed that both αA and αB crystallins exhibited oligomeric complexes in solution. Here, we present the structural characteristics of α-crystallin proteins from low to high temperature by combining circular dichroism (CD) and small-angle X-ray scattering (SAXS). Not only the CD data, but also SAXS data show that α-crystallin proteins exhibit transition behavior on conformation with temperature increasing. Although their protein sequences are highly conserved, the analysis of their thermal stability showed different properties in αA and αB crystallin. In this study, taken together, the data discussed were provided to demonstrate more insights into the chaperone-like activity of α-crystallin proteins.
Keywords: chaperone activity; circular dichroism; small-angle X-ray scattering; α-crystallin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Conformational and functional differences between recombinant human lens alphaA- and alphaB-crystallin.J Biol Chem. 1997 Mar 7;272(10):6220-5. doi: 10.1074/jbc.272.10.6220. J Biol Chem. 1997. PMID: 9045637
-
The IXI/V motif in the C-terminal extension of alpha-crystallins: alternative interactions and oligomeric assemblies.Mol Vis. 2004 Sep 8;10:655-62. Mol Vis. 2004. PMID: 15448619
-
Differential temperature-dependent chaperone-like activity of alphaA- and alphaB-crystallin homoaggregates.J Biol Chem. 1999 Dec 3;274(49):34773-8. doi: 10.1074/jbc.274.49.34773. J Biol Chem. 1999. PMID: 10574947
-
Proteostasis and the Regulation of Intra- and Extracellular Protein Aggregation by ATP-Independent Molecular Chaperones: Lens α-Crystallins and Milk Caseins.Acc Chem Res. 2018 Mar 20;51(3):745-752. doi: 10.1021/acs.accounts.7b00250. Epub 2018 Feb 14. Acc Chem Res. 2018. PMID: 29442498 Review.
-
sHSP in the eye lens: crystallin mutations, cataract and proteostasis.Int J Biochem Cell Biol. 2012 Oct;44(10):1687-97. doi: 10.1016/j.biocel.2012.02.015. Epub 2012 Mar 2. Int J Biochem Cell Biol. 2012. PMID: 22405853 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

