The Role of Bioenergetics in Neurodegeneration
- PMID: 36012480
- PMCID: PMC9409169
- DOI: 10.3390/ijms23169212
The Role of Bioenergetics in Neurodegeneration
Abstract
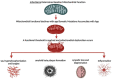
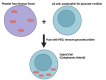
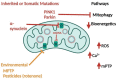
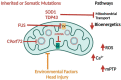
Bioenergetic and mitochondrial dysfunction are common hallmarks of neurodegenerative diseases. Decades of research describe how genetic and environmental factors initiate changes in mitochondria and bioenergetics across Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). Mitochondria control many cellular processes, including proteostasis, inflammation, and cell survival/death. These cellular processes and pathologies are common across neurodegenerative diseases. Evidence suggests that mitochondria and bioenergetic disruption may drive pathological changes, placing mitochondria as an upstream causative factor in neurodegenerative disease onset and progression. Here, we discuss evidence of mitochondrial and bioenergetic dysfunction in neurodegenerative diseases and address how mitochondria can drive common pathological features of these diseases.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; bioenergetics; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitochondrial dysfunction and oxidative stress in Alzheimer's disease, and Parkinson's disease, Huntington's disease and Amyotrophic Lateral Sclerosis -An updated review.Mitochondrion. 2023 Jul;71:83-92. doi: 10.1016/j.mito.2023.05.007. Epub 2023 Jun 1. Mitochondrion. 2023. PMID: 37269968 Review.
-
Mitochondria and neurodegeneration.Biosci Rep. 2007 Jun;27(1-3):87-104. doi: 10.1007/s10540-007-9038-z. Biosci Rep. 2007. PMID: 17486441 Review.
-
Mitochondrial diseases of the brain.Free Radic Biol Med. 2013 Oct;63:1-29. doi: 10.1016/j.freeradbiomed.2013.03.018. Epub 2013 Apr 6. Free Radic Biol Med. 2013. PMID: 23567191 Review.
-
The use of fibroblasts as a valuable strategy for studying mitochondrial impairment in neurological disorders.Transl Neurodegener. 2022 Jul 4;11(1):36. doi: 10.1186/s40035-022-00308-y. Transl Neurodegener. 2022. PMID: 35787292 Free PMC article. Review.
-
Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale.Cells. 2020 Dec 8;9(12):2642. doi: 10.3390/cells9122642. Cells. 2020. PMID: 33302607 Free PMC article.
Cited by
-
Nucleocytoplasmic transport rates are regulated by cellular processes that modulate GTP availability.bioRxiv [Preprint]. 2023 Dec 30:2023.12.29.573651. doi: 10.1101/2023.12.29.573651. bioRxiv. 2023. Update in: J Cell Biol. 2024 Jul 1;223(7):e202308152. doi: 10.1083/jcb.202308152. PMID: 38234722 Free PMC article. Updated. Preprint.
-
Mitochondrial Dysfunction as a Potential Mechanism Mediating Cardiac Comorbidities in Parkinson's Disease.Int J Mol Sci. 2024 Oct 12;25(20):10973. doi: 10.3390/ijms252010973. Int J Mol Sci. 2024. PMID: 39456761 Free PMC article. Review.
-
The Interplay of Mitochondrial Bioenergetics and Dopamine Agonists as an Effective Disease-Modifying Therapy for Parkinson's Disease.Mol Neurobiol. 2024 Oct;61(10):8086-8103. doi: 10.1007/s12035-024-04078-8. Epub 2024 Mar 11. Mol Neurobiol. 2024. PMID: 38468113 Review.
-
Molecular Mechanisms of the Anti-Inflammatory Effects of Epigallocatechin 3-Gallate (EGCG) in LPS-Activated BV-2 Microglia Cells.Brain Sci. 2023 Apr 7;13(4):632. doi: 10.3390/brainsci13040632. Brain Sci. 2023. PMID: 37190597 Free PMC article.
-
Nucleocytoplasmic transport rates are regulated by cellular processes that modulate GTP availability.J Cell Biol. 2024 Jul 1;223(7):e202308152. doi: 10.1083/jcb.202308152. Epub 2024 Apr 29. J Cell Biol. 2024. PMID: 38683248 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

