Cell Cycle Regulation by Integrin-Mediated Adhesion
- PMID: 36010598
- PMCID: PMC9406542
- DOI: 10.3390/cells11162521
Cell Cycle Regulation by Integrin-Mediated Adhesion
Abstract
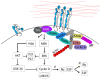
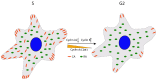
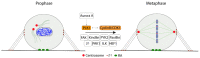
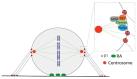
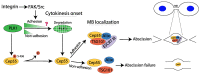
Cell cycle and cell adhesion are two interdependent cellular processes regulating each other, reciprocally, in every cell cycle phase. The cell adhesion to the extracellular matrix (ECM) via integrin receptors triggers signaling pathways required for the cell cycle progression; the passage from the G1 to S phase and the completion of cytokinesis are the best-understood events. Growing evidence, however, suggests more adhesion-dependent regulatory aspects of the cell cycle, particularly during G2 to M transition and early mitosis. Conversely, the cell cycle machinery regulates cell adhesion in manners recently shown driven mainly by cyclin-dependent kinase 1 (CDK1). This review summarizes the recent findings regarding the role of integrin-mediated cell adhesion and its downstream signaling components in regulating the cell cycle, emphasizing the cell cycle progression through the G2 and early M phases. Further investigations are required to raise our knowledge about the molecular mechanisms of crosstalk between cell adhesion and the cell cycle in detail.
Keywords: G2 phase; adhesion; cell cycle; integrin; mitosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cell cycle control by cell-matrix interactions.Curr Opin Cell Biol. 2024 Feb;86:102288. doi: 10.1016/j.ceb.2023.102288. Epub 2023 Dec 5. Curr Opin Cell Biol. 2024. PMID: 38056140 Review.
-
Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis.Cells. 2022 Apr 16;11(8):1360. doi: 10.3390/cells11081360. Cells. 2022. PMID: 35456039 Free PMC article.
-
Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells.Mol Cell Biol. 2004 Oct;24(19):8586-99. doi: 10.1128/MCB.24.19.8586-8599.2004. Mol Cell Biol. 2004. PMID: 15367678 Free PMC article.
-
Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody.Oncotarget. 2016 May 24;7(21):30820-30. doi: 10.18632/oncotarget.9003. Oncotarget. 2016. PMID: 27127172 Free PMC article.
-
Cell-Cycle-Dependent Regulation of Cell Adhesions: Adhering to the Schedule: Three papers reveal unexpected properties of adhesion structures as cells progress through the cell cycle.Bioessays. 2019 Jan;41(1):e1800165. doi: 10.1002/bies.201800165. Epub 2018 Nov 28. Bioessays. 2019. PMID: 30485463 Review.
Cited by
-
Inhibitory Effect of β-Sitosterol on the Ang II-Induced Proliferation of A7r5 Aortic Smooth Muscle Cells.Anal Cell Pathol (Amst). 2023 Nov 7;2023:2677020. doi: 10.1155/2023/2677020. eCollection 2023. Anal Cell Pathol (Amst). 2023. PMID: 38028434 Free PMC article.
-
Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Mar 14;8(1):116. doi: 10.1038/s41392-023-01343-5. Signal Transduct Target Ther. 2023. PMID: 36918530 Free PMC article. Review.
-
Immobilizing c(RGDfc) on the surface of metal-phenolic networks by thiol-click reaction for accelerating osteointegration of implant.Mater Today Bio. 2024 Mar 3;25:101017. doi: 10.1016/j.mtbio.2024.101017. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38495914 Free PMC article.
-
Cytoplasmic Polyadenylation Element Binding Protein 1 and Atherosclerosis: Prospective Target and New Insights.Curr Vasc Pharmacol. 2024;22(2):95-105. doi: 10.2174/0115701611258090231221082502. Curr Vasc Pharmacol. 2024. PMID: 38284693 Review.
-
The interplay between cell wall integrity and cell cycle progression in plants.Plant Mol Biol. 2023 Dec;113(6):367-382. doi: 10.1007/s11103-023-01394-w. Epub 2023 Dec 13. Plant Mol Biol. 2023. PMID: 38091166 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

