Simple Detection of Unstained Live Senescent Cells with Imaging Flow Cytometry
- PMID: 36010584
- PMCID: PMC9406876
- DOI: 10.3390/cells11162506
Simple Detection of Unstained Live Senescent Cells with Imaging Flow Cytometry
Abstract
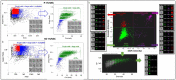
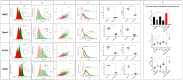
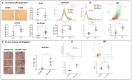
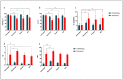
Cellular senescence is a hallmark of aging and a promising target for therapeutic approaches. The identification of senescent cells requires multiple biomarkers and complex experimental procedures, resulting in increased variability and reduced sensitivity. Here, we propose a simple and broadly applicable imaging flow cytometry (IFC) method. This method is based on measuring autofluorescence and morphological parameters and on applying recent artificial intelligence (AI) and machine learning (ML) tools. We show that the results of this method are superior to those obtained measuring the classical senescence marker, senescence-associated beta-galactosidase (SA-β-Gal). We provide evidence that this method has the potential for diagnostic or prognostic applications as it was able to detect senescence in cardiac pericytes isolated from the hearts of patients affected by end-stage heart failure. We additionally demonstrate that it can be used to quantify senescence "in vivo" and can be used to evaluate the effects of senolytic compounds. We conclude that this method can be used as a simple and fast senescence assay independently of the origin of the cells and the procedure to induce senescence.
Keywords: artificial intelligence and machine learning; cellular senescence; imaging flow cytometry; replicative senescence; senolytics.
Conflict of interest statement
Alessandro Serra disclaims that he is affiliate of Luminex, whose portfolio comprises the imaging flow cytometry and software of Amnis used in this study. All other authors declare no conflicts of interest.
Figures
Similar articles
-
Far-red Fluorescent Senescence-associated β-Galactosidase Probe for Identification and Enrichment of Senescent Tumor Cells by Flow Cytometry.J Vis Exp. 2022 Sep 13;(187):10.3791/64176. doi: 10.3791/64176. J Vis Exp. 2022. PMID: 36190263 Free PMC article.
-
Multiparameter flow cytometric detection and quantification of senescent cells in vitro.Biogerontology. 2020 Dec;21(6):773-786. doi: 10.1007/s10522-020-09893-9. Epub 2020 Aug 10. Biogerontology. 2020. PMID: 32776262 Free PMC article.
-
Detection of Senescent Cells by Extracellular Markers Using a Flow Cytometry-Based Approach.Methods Mol Biol. 2017;1534:147-153. doi: 10.1007/978-1-4939-6670-7_14. Methods Mol Biol. 2017. PMID: 27812876
-
Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay.Methods Mol Biol. 2007;371:21-31. doi: 10.1007/978-1-59745-361-5_3. Methods Mol Biol. 2007. PMID: 17634571 Review.
-
Cellular senescence in cardiac diseases.J Cardiol. 2019 Oct;74(4):313-319. doi: 10.1016/j.jjcc.2019.05.002. Epub 2019 Jun 12. J Cardiol. 2019. PMID: 31202488 Review.
Cited by
-
The role of cellular senescence in ovarian aging.NPJ Aging. 2024 Jul 20;10(1):35. doi: 10.1038/s41514-024-00157-1. NPJ Aging. 2024. PMID: 39033161 Free PMC article. Review.
-
Comparative analysis of feature-based ML and CNN for binucleated erythroblast quantification in myelodysplastic syndrome patients using imaging flow cytometry data.Sci Rep. 2024 Apr 23;14(1):9349. doi: 10.1038/s41598-024-59875-x. Sci Rep. 2024. PMID: 38654058 Free PMC article.
-
Cellular senescence in cancer: clinical detection and prognostic implications.J Exp Clin Cancer Res. 2022 Dec 27;41(1):360. doi: 10.1186/s13046-022-02555-3. J Exp Clin Cancer Res. 2022. PMID: 36575462 Free PMC article. Review.
-
Exogenous Iron Induces Mitochondrial Lipid Peroxidation, Lipofuscin Accumulation, and Ferroptosis in H9c2 Cardiomyocytes.Biomolecules. 2024 Jun 19;14(6):730. doi: 10.3390/biom14060730. Biomolecules. 2024. PMID: 38927133 Free PMC article.
-
Enhancing Immune Responses against SARS-CoV-2 Variants in Aged Mice with INDUK: A Chimeric DNA Vaccine Encoding the Spike S1-TM Subunits.ACS Omega. 2024 Jul 30;9(32):34624-34635. doi: 10.1021/acsomega.4c03285. eCollection 2024 Aug 13. ACS Omega. 2024. PMID: 39157118 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

