Development of Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Detecting Feline Coronavirus
- PMID: 36009664
- PMCID: PMC9405184
- DOI: 10.3390/ani12162075
Development of Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Detecting Feline Coronavirus
Abstract
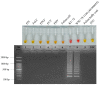
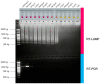
Feline infectious peritonitis (FIP) is a worldwide fatal disease caused by a mutant feline coronavirus (FCoV). Simple and efficient molecular detection methods are needed. Here, sensitive, specific, rapid, and reliable colorimetric reverse transcription loop-mediated isothermal amplification (RT-LAMP) was developed to detect the ORF1a/1b gene of FCoV from cats with suspected FIP using neutral red as an indicator. Novel LAMP primers were specifically designed based on the gene of interest. The isothermal assay could visually detect FCoV at 58 °C for 50 min. The RT-LAMP assay was highly specific and had no cross-reactivity with other related feline viruses. The detection limit of FCoV detection by RT-LAMP was 20 fg/µL. A blind clinical test (n = 81) of the developed RT-LAMP procedure was in good agreement with the conventional PCR method. In the light of its performance specificity, sensitivity, and easy visualization, this neutral-red-based RT-LAMP approach would be a fruitful alternative molecular diagnostic tool for veterinary inspection of FCoV when combined with nucleotide sequencing or specific PCR to affirm the highly virulent FIP-associated FCoV.
Keywords: feline infectious peritonitis; molecular diagnosis; reverse transcription loop-mediated isothermal amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Detection of feline Coronavirus in effusions of cats with and without feline infectious peritonitis using loop-mediated isothermal amplification.J Virol Methods. 2018 Jun;256:32-36. doi: 10.1016/j.jviromet.2018.03.003. Epub 2018 Mar 11. J Virol Methods. 2018. PMID: 29540320 Free PMC article.
-
Reverse transcriptase loop-mediated isothermal amplification for the detection of feline coronavirus.J Virol Methods. 2017 May;243:105-108. doi: 10.1016/j.jviromet.2017.01.009. Epub 2017 Jan 18. J Virol Methods. 2017. PMID: 28109842 Free PMC article.
-
Molecular Detection of Feline Coronavirus Based on Recombinase Polymerase Amplification Assay.Pathogens. 2021 Sep 25;10(10):1237. doi: 10.3390/pathogens10101237. Pathogens. 2021. PMID: 34684186 Free PMC article.
-
Feline infectious peritonitis (FIP) and coronavirus disease 19 (COVID-19): Are they similar?Transbound Emerg Dis. 2021 Jul;68(4):1786-1799. doi: 10.1111/tbed.13856. Epub 2020 Oct 20. Transbound Emerg Dis. 2021. PMID: 32985113 Free PMC article. Review.
-
Colorimetric biosensors for point-of-care virus detections.Mater Sci Energy Technol. 2020;3:237-249. doi: 10.1016/j.mset.2019.10.002. Epub 2019 Oct 23. Mater Sci Energy Technol. 2020. PMID: 33604529 Free PMC article. Review.
Cited by
-
Detection of feline immunodeficiency virus by neutral red-based loop-mediated isothermal amplification assay.Vet World. 2024 Jan;17(1):72-81. doi: 10.14202/vetworld.2024.72-81. Epub 2024 Jan 8. Vet World. 2024. PMID: 38406374 Free PMC article.
-
Comparison of PCR, Nested PCR, and RT-LAMP for Rapid Detection of Feline Calicivirus Infection in Clinical Samples.Animals (Basel). 2024 Aug 22;14(16):2432. doi: 10.3390/ani14162432. Animals (Basel). 2024. PMID: 39199965 Free PMC article.
-
Feline infectious peritonitis: A comprehensive evaluation of clinical manifestations, laboratory diagnosis, and therapeutic approaches.J Adv Vet Anim Res. 2024 Mar 12;11(1):19-26. doi: 10.5455/javar.2024.k742. eCollection 2024 Mar. J Adv Vet Anim Res. 2024. PMID: 38680809 Free PMC article.
-
The first study on clinicopathological changes in cats with feline infectious peritonitis with and without retrovirus coinfection.Vet World. 2023 Apr;16(4):820-827. doi: 10.14202/vetworld.2023.820-827. Epub 2023 Apr 20. Vet World. 2023. PMID: 37235153 Free PMC article.
-
Domestic and wild animal samples and diagnostic testing for SARS-CoV-2.Vet Q. 2023 Dec;43(1):1-11. doi: 10.1080/01652176.2023.2263864. Epub 2023 Oct 26. Vet Q. 2023. PMID: 37779468 Free PMC article. Review.
References
-
- Mavrodiev E.V., Tursky M.L., Mavrodiev N.E., Ebach M.C., Williams D.M. On Classification and Taxonomy of Coronaviruses (Riboviria, Nidovirales, Coronaviridae) with Special Focus on Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-Cov-2) Short Title: On the Classification of Coronaviruses. bioRxiv. 2020 doi: 10.1101/2020.10.17.343749. - DOI
Grants and funding
- RUP2.2/Con-CASAF PD 03, KURDI(FF(KU)66)/the Office of the Ministry of Higher Education, Science, Research and Innovation; and the Thailand Science Research and Innovation through the Kasetsart University Rein-venting University Program 2021, the Kasetsart University Research and Development Ins
- R. Thanawongnuwech NRCT Senior scholar 2022 #N42A650553/National Research Council of Thailand (NRCT)
LinkOut - more resources
Full Text Sources
Miscellaneous

