Pharmacological Properties and Molecular Targets of Alisol Triterpenoids from Alismatis Rhizoma
- PMID: 36009492
- PMCID: PMC9406200
- DOI: 10.3390/biomedicines10081945
Pharmacological Properties and Molecular Targets of Alisol Triterpenoids from Alismatis Rhizoma
Abstract
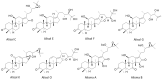
More than 100 protostane triterpenoids have been isolated from the dried rhizomes of Alisma species, designated Alismatis rhizoma (AR), commonly used in Asian traditional medicine to treat inflammatory and vascular diseases. The main products are the alisols, with the lead compounds alisol-A/-B and their acetate derivatives being the most abundant products in the plant and the best-known bioactive products. The pharmacological effects of Ali-A, Ali-A 24-acetate, Ali-B, Ali-B 23-acetate, and derivatives have been analyzed to provide an overview of the medicinal properties, signaling pathways, and molecular targets at the origin of those activities. Diverse protein targets have been proposed for these natural products, including the farnesoid X receptor, soluble epoxide hydrolase, and other enzymes (AMPK, HCE-2) and functional proteins (YAP, LXR) at the origin of the anti-atherosclerosis, anti-inflammatory, antioxidant, anti-fibrotic, and anti-proliferative activities. Activities were classified in two groups. The lipid-lowering and anti-atherosclerosis effects benefit from robust in vitro and in vivo data (group 1). The anticancer effects of alisols have been largely reported, but, essentially, studies using tumor cell lines and solid in vivo data are lacking (group 2). The survey shed light on the pharmacological properties of alisol triterpenoids frequently found in traditional phytomedicines.
Keywords: Alismatis rhizoma; alisol; cancer; inflammation; molecular targets; pharmacology; protostane triterpenoids.
Conflict of interest statement
The author declares no conflict of interest.
Figures
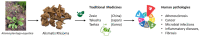
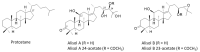

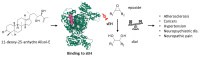

Similar articles
-
Promising Anticancer Activities of Alismatis rhizome and Its Triterpenes via p38 and PI3K/Akt/mTOR Signaling Pathways.Nutrients. 2021 Jul 18;13(7):2455. doi: 10.3390/nu13072455. Nutrients. 2021. PMID: 34371964 Free PMC article. Review.
-
Two Undescribed Protostane Triterpenoids from the Rhizome of Alisma plantago-aquatica.Chem Biodivers. 2024 Mar;21(3):e202301631. doi: 10.1002/cbdv.202301631. Epub 2024 Feb 8. Chem Biodivers. 2024. PMID: 38205915
-
Effects of protostane-type triterpenoids on the 5-HT3A receptor-mediated ion current in Xenopus oocytes.Brain Res. 2010 May 17;1331:20-7. doi: 10.1016/j.brainres.2010.03.041. Epub 2010 Mar 20. Brain Res. 2010. PMID: 20307506
-
Mechanism of triterpenoids from Alismatis Rhizoma against liver fibrosis based on an integrated approach using network pharmacology, molecular docking, and luciferase assay.Nat Prod Res. 2023 Nov-Dec;37(22):3826-3831. doi: 10.1080/14786419.2022.2149520. Epub 2022 Nov 26. Nat Prod Res. 2023. PMID: 36434777
-
Therapeutic potential of Rhizoma Alismatis: a review on ethnomedicinal application, phytochemistry, pharmacology, and toxicology.Ann N Y Acad Sci. 2017 Aug;1401(1):90-101. doi: 10.1111/nyas.13381. Epub 2017 Jun 29. Ann N Y Acad Sci. 2017. PMID: 28662316 Review.
Cited by
-
Neurovascular protection of alisol A on cerebral ischemia mice through activating the AKT/GSK3β pathway.Aging (Albany NY). 2023 Oct 26;15(20):11639-11653. doi: 10.18632/aging.205151. Epub 2023 Oct 26. Aging (Albany NY). 2023. PMID: 37889534 Free PMC article.
-
Properties and Fungal Communities of Different Soils for Growth of the Medicinal Asian Water Plantain, Alisma orientale, in Fujian, China.J Fungi (Basel). 2024 Feb 29;10(3):187. doi: 10.3390/jof10030187. J Fungi (Basel). 2024. PMID: 38535196 Free PMC article.
-
Alisol A, the Eye-Entering Ingredient of Alisma orientale, Relieves Macular Edema Through TNF-α as Revealed by UPLC-Triple-TOF/MS, Network Pharmacology, and Zebrafish Verification.Drug Des Devel Ther. 2024 Jul 30;18:3361-3382. doi: 10.2147/DDDT.S468119. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39100223 Free PMC article.
-
The roles of serine hydrolases and serum albumin in alisol B 23-acetate hydrolysis in humans.Front Pharmacol. 2023 Apr 6;14:1160665. doi: 10.3389/fphar.2023.1160665. eCollection 2023. Front Pharmacol. 2023. PMID: 37089921 Free PMC article.
-
Alisma Orientalis Extract Ameliorates Hepatic Iron Deregulation in MAFLD Mice via FXR-Mediated Gene Repression.Nutrients. 2024 Jul 15;16(14):2272. doi: 10.3390/nu16142272. Nutrients. 2024. PMID: 39064715 Free PMC article.
References
-
- Liu S.S., Wei S.H., Zhu J.J., Zhang W.W., Jiao S., Guo J., Yan L.H., Wang Z.M. Herbal textural research, morphologic characteristics and DNA barcoding of botanical origins of Alismatis Rhizoma. Zhongguo Zhong Yao Za Zhi. 2020;45:1536–1544. - PubMed
-
- Liu S.S., Guo J., Li Z.A., Tian S.S., Zhu J.J., Yan L.H., Wang Z.M., Gao L. Advances in studies on chemical compositions of Alismatis Rhizoma and their biological activities. Zhongguo Zhong Yao Za Zhi. 2020;45:1578–1595. - PubMed
-
- Zhang J., Jin Q., Wu W., Jin X., An Y., Liu C., Wei W., Li Z., Yao C., Yao S., et al. “Force iteration molecular designing” strategy for the systematic characterization and discovery of new protostane triterpenoids from Alisma Rhizoma by UHPLC/LTQ-Orbitrap-MS. Anal. Bioanal. Chem. 2021;413:1749–1764. doi: 10.1007/s00216-020-03145-y. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

