SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance
- PMID: 36009329
- PMCID: PMC9404744
- DOI: 10.3390/antiox11081611
SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance
Abstract
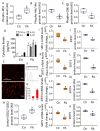
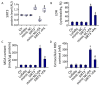
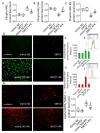
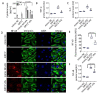
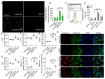
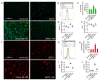
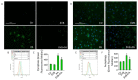
Emerging evidence indicates that defects in sirtuin signaling contribute to impaired glucose and lipid metabolism, resulting in insulin resistance (IR) and endothelial dysfunction. Here, we examined the effects of palmitic acid (PA) treatment on mitochondrial sirtuins (SIRT2, SIRT3, SIRT4, and SIRT5) and oxidative homeostasis in human endothelial cells (TeloHAEC). Results showed that treatment for 48 h with PA (0.5 mM) impaired cell viability, induced loss of insulin signaling, imbalanced the oxidative status (p < 0.001), and caused negative modulation of sirtuin protein and mRNA expression, with a predominant effect on SIRT3 (p < 0.001). Restoration of SIRT3 levels by mimic transfection (SIRT3+) suppressed the PA-induced autophagy (mimic NC+PA) (p < 0.01), inflammation, and pyroptosis (p < 0.01) mediated by the NLRP3/caspase-1 axis. Moreover, the unbalanced endothelial redox state induced by PA was counteracted by the antioxidant δ-valerobetaine (δVB), which was able to upregulate protein and mRNA expression of sirtuins, reduce reactive oxygen species (ROS) accumulation, and decrease cell death. Overall, results support the central role of SIRT3 in maintaining the endothelial redox homeostasis under IR and unveil the potential of the antioxidant δVB in enhancing the defense against IR-related injuries.
Keywords: SIRT3; endothelial cells; insulin resistance; mitochondria; δ-valerobetaine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Screening Analysis of Sirtuins Family Expression on Anti-Inflammation of Resveratrol in Endothelial Cells.Med Sci Monit. 2019 Jun 3;25:4137-4148. doi: 10.12659/MSM.913240. Med Sci Monit. 2019. PMID: 31158122 Free PMC article.
-
Mitochondrial sirtuins in the rat adrenal gland: location within the glands of males and females, hormonal and developmental regulation of gene expressions.Folia Histochem Cytobiol. 2017;55(4):190-202. doi: 10.5603/FHC.a2017.0020. Epub 2017 Dec 20. Folia Histochem Cytobiol. 2017. PMID: 29261224
-
Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5.J Mol Biol. 2008 Oct 10;382(3):790-801. doi: 10.1016/j.jmb.2008.07.048. Epub 2008 Jul 25. J Mol Biol. 2008. PMID: 18680753
-
Roles of Mitochondrial Sirtuins in Mitochondrial Function, Redox Homeostasis, Insulin Resistance and Type 2 Diabetes.Int J Mol Sci. 2020 Jul 24;21(15):5266. doi: 10.3390/ijms21155266. Int J Mol Sci. 2020. PMID: 32722262 Free PMC article. Review.
-
Mitochondrial Sirtuins in Reproduction.Antioxidants (Basel). 2021 Jun 29;10(7):1047. doi: 10.3390/antiox10071047. Antioxidants (Basel). 2021. PMID: 34209765 Free PMC article. Review.
Cited by
-
Physiological and pathological characteristics of vascular endothelial injury in diabetes and the regulatory mechanism of autophagy.Front Endocrinol (Lausanne). 2023 Jun 27;14:1191426. doi: 10.3389/fendo.2023.1191426. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37441493 Free PMC article. Review.
-
Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State.Antioxidants (Basel). 2023 Jun 20;12(6):1311. doi: 10.3390/antiox12061311. Antioxidants (Basel). 2023. PMID: 37372041 Free PMC article.
-
MiR-15b-5p and PCSK9 inhibition reduces lipopolysaccharide-induced endothelial dysfunction by targeting SIRT4.Cell Mol Biol Lett. 2023 Aug 16;28(1):66. doi: 10.1186/s11658-023-00482-5. Cell Mol Biol Lett. 2023. PMID: 37587410 Free PMC article.
-
MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction.Int J Mol Sci. 2024 May 7;25(10):5087. doi: 10.3390/ijms25105087. Int J Mol Sci. 2024. PMID: 38791128 Free PMC article.
-
Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer.Cells. 2024 Apr 9;13(8):663. doi: 10.3390/cells13080663. Cells. 2024. PMID: 38667278 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

