A genome sequencing system for universal newborn screening, diagnosis, and precision medicine for severe genetic diseases
- PMID: 36007526
- PMCID: PMC9502059
- DOI: 10.1016/j.ajhg.2022.08.003
A genome sequencing system for universal newborn screening, diagnosis, and precision medicine for severe genetic diseases
Abstract
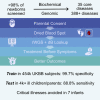
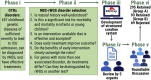
Newborn screening (NBS) dramatically improves outcomes in severe childhood disorders by treatment before symptom onset. In many genetic diseases, however, outcomes remain poor because NBS has lagged behind drug development. Rapid whole-genome sequencing (rWGS) is attractive for comprehensive NBS because it concomitantly examines almost all genetic diseases and is gaining acceptance for genetic disease diagnosis in ill newborns. We describe prototypic methods for scalable, parentally consented, feedback-informed NBS and diagnosis of genetic diseases by rWGS and virtual, acute management guidance (NBS-rWGS). Using established criteria and the Delphi method, we reviewed 457 genetic diseases for NBS-rWGS, retaining 388 (85%) with effective treatments. Simulated NBS-rWGS in 454,707 UK Biobank subjects with 29,865 pathogenic or likely pathogenic variants associated with 388 disorders had a true negative rate (specificity) of 99.7% following root cause analysis. In 2,208 critically ill children with suspected genetic disorders and 2,168 of their parents, simulated NBS-rWGS for 388 disorders identified 104 (87%) of 119 diagnoses previously made by rWGS and 15 findings not previously reported (NBS-rWGS negative predictive value 99.6%, true positive rate [sensitivity] 88.8%). Retrospective NBS-rWGS diagnosed 15 children with disorders that had been undetected by conventional NBS. In 43 of the 104 children, had NBS-rWGS-based interventions been started on day of life 5, the Delphi consensus was that symptoms could have been avoided completely in seven critically ill children, mostly in 21, and partially in 13. We invite groups worldwide to refine these NBS-rWGS conditions and join us to prospectively examine clinical utility and cost effectiveness.
Keywords: UK Biobank; clinical decision support; clinical utility; diagnosis; diagnostic odyssey; gene therapy; genetic disease; newborn screening; orphan drug; rapid whole-genome sequencing; sensitivity; specificity; virtual management guidance.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.P.H., C.M.K., S.S.M., and D.T. are employees and shareholders of Illumina, Inc. G.D,A., B.M., S.L., and T.D. are employees and shareholders of Alexion Pharmaceuticals. E.F. and M.G.R. are employees and shareholders of Fabric Genomics, Inc. M.K. and S.S. are employees and shareholders of Genomenon, Inc. C.K., G.P., S.S., S.P., and A.R.W. are employees and shareholders of TileDB, Inc. S.K. is an employee and shareholder of Luna PBC, Inc. S.K. has filed a patent related to this work.
Figures


Comment in
-
Progress in expanding newborn screening in the United States.Am J Hum Genet. 2023 Jun 1;110(6):1015-1016. doi: 10.1016/j.ajhg.2023.05.002. Am J Hum Genet. 2023. PMID: 37267896 Free PMC article. No abstract available.
Similar articles
-
Evaluating parents' decisions about next-generation sequencing for their child in the NC NEXUS (North Carolina Newborn Exome Sequencing for Universal Screening) study: a randomized controlled trial protocol.Trials. 2018 Jun 28;19(1):344. doi: 10.1186/s13063-018-2686-4. Trials. 2018. PMID: 29950170 Free PMC article.
-
Dispatches from Biotech beginning BeginNGS: Rapid newborn genome sequencing to end the diagnostic and therapeutic odyssey.Am J Med Genet C Semin Med Genet. 2022 Jun;190(2):243-256. doi: 10.1002/ajmg.c.32005. Epub 2022 Oct 11. Am J Med Genet C Semin Med Genet. 2022. PMID: 36218021 Free PMC article.
-
Project Baby Bear: Rapid precision care incorporating rWGS in 5 California children's hospitals demonstrates improved clinical outcomes and reduced costs of care.Am J Hum Genet. 2021 Jul 1;108(7):1231-1238. doi: 10.1016/j.ajhg.2021.05.008. Epub 2021 Jun 4. Am J Hum Genet. 2021. PMID: 34089648 Free PMC article.
-
Newborn Screening in the Era of Precision Medicine.Adv Exp Med Biol. 2017;1005:47-61. doi: 10.1007/978-981-10-5717-5_3. Adv Exp Med Biol. 2017. PMID: 28916928 Review.
-
Insights into National Laboratory Newborn Screening and Future Prospects.Medicina (Kaunas). 2022 Feb 11;58(2):272. doi: 10.3390/medicina58020272. Medicina (Kaunas). 2022. PMID: 35208595 Free PMC article. Review.
Cited by
-
Scaling genetic resources: New paradigms for diagnosis and treatment of rare genetic disease.Am J Med Genet C Semin Med Genet. 2023 Mar;193(1):77-86. doi: 10.1002/ajmg.c.32016. Epub 2022 Nov 30. Am J Med Genet C Semin Med Genet. 2023. PMID: 36448938 Free PMC article.
-
Opportunities and challenges for newborn screening and early diagnosis of rare diseases in Latin America.Front Genet. 2022 Dec 8;13:1053559. doi: 10.3389/fgene.2022.1053559. eCollection 2022. Front Genet. 2022. PMID: 36568372 Free PMC article. Review.
-
The Development of an Infrastructure to Facilitate the Use of Whole Genome Sequencing for Population Health.J Pers Med. 2022 Nov 8;12(11):1867. doi: 10.3390/jpm12111867. J Pers Med. 2022. PMID: 36579594 Free PMC article.
-
Scalable, high quality, whole genome sequencing from archived, newborn, dried blood spots.NPJ Genom Med. 2023 Feb 14;8(1):5. doi: 10.1038/s41525-023-00349-w. NPJ Genom Med. 2023. PMID: 36788231 Free PMC article.
-
Precision medicine in Asia enhanced by next-generation sequencing: Implications for Thailand through a scoping review and interview study.Clin Transl Sci. 2024 Jun;17(6):e13868. doi: 10.1111/cts.13868. Clin Transl Sci. 2024. PMID: 38924657 Free PMC article. Review.
References
-
- Therrell B.L., Padilla C.D., Loeber J.G., Kneisser I., Saadallah A., Borrajo G.J.C., Adams J. Current status of newborn screening worldwide. Semin. Perinatol. 2015;39:171–187. - PubMed
-
- American Academy of Pediatrics Newborn Screening Task Force Newborn screening: a blueprint for the future. Pediatrics. 2000;106(Suppl. 2):383–427. - PubMed
-
- Sontag M.K., Yusuf C., Grosse S.D., Edelman S., Miller J.I., McKasson S., Kellar-Guenther Y., Gaffney M., Hinton C.F., Cuthbert C., et al. Infants with Congenital Disorders Identified Through Newborn Screening — United States, 2015–2017. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1265–1268. - PMC - PubMed
-
- Centers for Disease Control and Prevention CDC Impact of expanded newborn screening—United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 2008;57:1012–1015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

