The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors
- PMID: 36005489
- PMCID: PMC9409833
- DOI: 10.3390/md20080488
The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors
Abstract
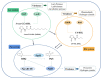
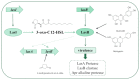
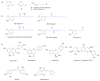
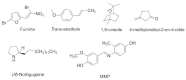
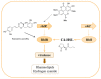
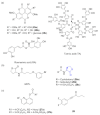
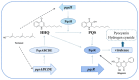
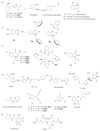
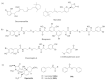
The survival selection pressure caused by antibiotic-mediated bactericidal and bacteriostatic activity is one of the important inducements for bacteria to develop drug resistance. Bacteria gain drug resistance through spontaneous mutation so as to achieve the goals of survival and reproduction. Quorum sensing (QS) is an intercellular communication system based on cell density that can regulate bacterial virulence and biofilm formation. The secretion of more than 30 virulence factors of P. aeruginosa is controlled by QS, and the formation and diffusion of biofilm is an important mechanism causing the multidrug resistance of P. aeruginosa, which is also closely related to the QS system. There are three main QS systems in P. aeruginosa: las system, rhl system, and pqs system. Quorum-sensing inhibitors (QSIs) can reduce the toxicity of bacteria without affecting the growth and enhance the sensitivity of bacterial biofilms to antibiotic treatment. These characteristics make QSIs a popular topic for research and development in the field of anti-infection. This paper reviews the research progress of the P. aeruginosa quorum-sensing system and QSIs, targeting three QS systems, which will provide help for the future research and development of novel quorum-sensing inhibitors.
Keywords: Pseudomonas aeruginosa; inhibitor; quorum sensing; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Inhibition of Virulence Factors and Biofilm Formation by Wogonin Attenuates Pathogenicity of Pseudomonas aeruginosa PAO1 via Targeting pqs Quorum-Sensing System.Int J Mol Sci. 2021 Nov 24;22(23):12699. doi: 10.3390/ijms222312699. Int J Mol Sci. 2021. PMID: 34884499 Free PMC article.
-
Evidence for Complex Interplay between Quorum Sensing and Antibiotic Resistance in Pseudomonas aeruginosa.Microbiol Spectr. 2022 Dec 21;10(6):e0126922. doi: 10.1128/spectrum.01269-22. Epub 2022 Oct 31. Microbiol Spectr. 2022. PMID: 36314960 Free PMC article.
-
Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors.Appl Microbiol Biotechnol. 2019 Apr;103(8):3521-3535. doi: 10.1007/s00253-019-09618-0. Epub 2019 Mar 9. Appl Microbiol Biotechnol. 2019. PMID: 30852658 Free PMC article.
-
Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors.Microb Pathog. 2021 Jun;155:104912. doi: 10.1016/j.micpath.2021.104912. Epub 2021 Apr 28. Microb Pathog. 2021. PMID: 33932548 Review.
-
Strategies for quorum sensing inhibition as a tool for controlling Pseudomonas aeruginosa infections.Int J Antimicrob Agents. 2024 Nov;64(5):107323. doi: 10.1016/j.ijantimicag.2024.107323. Epub 2024 Sep 4. Int J Antimicrob Agents. 2024. PMID: 39242051 Review.
Cited by
-
Strategies and materials for the prevention and treatment of biofilms.Mater Today Bio. 2023 Oct 2;23:100827. doi: 10.1016/j.mtbio.2023.100827. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37859998 Free PMC article. Review.
-
Effect of Biogenic Silver Nanoparticles on the Quorum-Sensing System of Pseudomonas aeruginosa PAO1 and PA14.Microorganisms. 2022 Aug 30;10(9):1755. doi: 10.3390/microorganisms10091755. Microorganisms. 2022. PMID: 36144357 Free PMC article.
-
The Role of Quorum Sensing Molecules in Bacterial-Plant Interactions.Metabolites. 2023 Jan 10;13(1):114. doi: 10.3390/metabo13010114. Metabolites. 2023. PMID: 36677039 Free PMC article. Review.
-
Carvacrol Inhibits Quorum Sensing in Opportunistic Bacterium Aeromonas hydrophila.Microorganisms. 2023 Aug 7;11(8):2027. doi: 10.3390/microorganisms11082027. Microorganisms. 2023. PMID: 37630587 Free PMC article.
-
Multidimensional Criteria for Virtual Screening of PqsR Inhibitors Based on Pharmacophore, Docking, and Molecular Dynamics.Int J Mol Sci. 2024 Feb 3;25(3):1869. doi: 10.3390/ijms25031869. Int J Mol Sci. 2024. PMID: 38339148 Free PMC article.
References
-
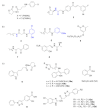
- Nath B.N., Iskander G.M., Mielczarek M., Yu T.T., Black D.S., Kumar N. Alkyne-substituted fimbrolide analogues as novel bacterial quorum-sensing inhibitors. Aust. J. Chem. 2018;71:708–715.
-
- Swift S., Allan Downie J., Whitehead N.A. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 2001;45:199–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

