Mucosal immunization with an adenoviral vector vaccine confers superior protection against RSV compared to natural immunity
- PMID: 36003372
- PMCID: PMC9394428
- DOI: 10.3389/fimmu.2022.920256
Mucosal immunization with an adenoviral vector vaccine confers superior protection against RSV compared to natural immunity
Abstract
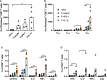
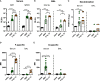
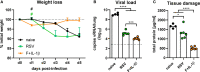
Respiratory syncytial virus (RSV) infections are the leading cause of severe respiratory illness in early infancy. Although the majority of children and adults mount immune responses against RSV, recurrent infections are frequent throughout life. Humoral and cellular responses contribute to an effective immunity but also their localization at respiratory mucosae is increasingly recognized as an important factor. In the present study, we evaluate a mucosal vaccine based on an adenoviral vector encoding for the RSV fusion protein (Ad-F), and we investigate two genetic adjuvant candidates that encode for Interleukin (IL)-1β and IFN-β promoter stimulator I (IPS-1), respectively. While vaccination with Ad-F alone was immunogenic, the inclusion of Ad-IL-1β increased F-specific mucosal immunoglobulin A (IgA) and tissue-resident memory T cells (TRM). Consequently, immunization with Ad-F led to some control of virus replication upon RSV infection, but Ad-F+Ad-IL-1β was the most effective vaccine strategy in limiting viral load and weight loss. Subsequently, we compared the Ad-F+Ad-IL-1β-induced immunity with that provoked by a primary RSV infection. Systemic F-specific antibody responses were higher in immunized than in previously infected mice. However, the primary infection provoked glycoprotein G-specific antibodies as well eventually leading to similar neutralization titers in both groups. In contrast, mucosal antibody levels were low after infection, whereas mucosal immunization raised robust F-specific responses including IgA. Similarly, vaccination generated F-specific TRM more efficiently compared to a primary RSV infection. Although the primary infection resulted in matrix protein 2 (M2)-specific T cells as well, they did not reach levels of F-specific immunity in the vaccinated group. Moreover, the infection-induced T cell response was less biased towards TRM compared to vaccine-induced immunity. Finally, our vaccine candidate provided superior protection against RSV infection compared to a primary infection as indicated by reduced weight loss, virus replication, and tissue damage. In conclusion, our mucosal vaccine candidate Ad-F+Ad-IL-1β elicits stronger mucosal immune responses and a more effective protection against RSV infection than natural immunity generated by a previous infection. Harnessing mucosal immune responses by next-generation vaccines is therefore a promising option to establish effective RSV immunity and thereby tackle a major cause of infant hospitalization.
Keywords: IgA; RSV (respiratory syncytial virus); TRM; adenoviral (Ad) vector; mucosal; natural immunity; tissue-resident memory T cells; vaccine.
Copyright © 2022 Maier, Fuchs, Irrgang, Wißing, Beyerlein, Tenbusch and Lapuente.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Filling two needs with one deed: a combinatory mucosal vaccine against influenza A virus and respiratory syncytial virus.Front Immunol. 2024 Jun 21;15:1376395. doi: 10.3389/fimmu.2024.1376395. eCollection 2024. Front Immunol. 2024. PMID: 38975350 Free PMC article.
-
Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats.Vaccine. 2015 Oct 5;33(41):5406-5414. doi: 10.1016/j.vaccine.2015.08.056. Epub 2015 Aug 28. Vaccine. 2015. PMID: 26319741
-
Intranasal immunization with a helper-dependent adenoviral vector expressing the codon-optimized fusion glycoprotein of human respiratory syncytial virus elicits protective immunity in BALB/c mice.Virol J. 2013 Jun 7;10:183. doi: 10.1186/1743-422X-10-183. Virol J. 2013. PMID: 23742026 Free PMC article.
-
The third pandemic: The respiratory syncytial virus landscape and specific considerations for the allergist/immunologist.Allergy Asthma Proc. 2023 Jul 26;44(4):220-228. doi: 10.2500/aap.2023.44.230030. Allergy Asthma Proc. 2023. PMID: 37236777 Review.
-
Induction and Subversion of Human Protective Immunity: Contrasting Influenza and Respiratory Syncytial Virus.Front Immunol. 2018 Mar 2;9:323. doi: 10.3389/fimmu.2018.00323. eCollection 2018. Front Immunol. 2018. PMID: 29552008 Free PMC article. Review.
Cited by
-
Filling two needs with one deed: a combinatory mucosal vaccine against influenza A virus and respiratory syncytial virus.Front Immunol. 2024 Jun 21;15:1376395. doi: 10.3389/fimmu.2024.1376395. eCollection 2024. Front Immunol. 2024. PMID: 38975350 Free PMC article.
-
Evaluation of adenoviral vector Ad19a encoding RSV-F as novel vaccine against respiratory syncytial virus.NPJ Vaccines. 2024 Oct 29;9(1):205. doi: 10.1038/s41541-024-01001-z. NPJ Vaccines. 2024. PMID: 39472590 Free PMC article.
-
Mucosal tumor vaccination delivering endogenous tumor antigens protects against pulmonary breast cancer metastases.J Immunother Cancer. 2024 Mar 7;12(3):e008652. doi: 10.1136/jitc-2023-008652. J Immunother Cancer. 2024. PMID: 38458636 Free PMC article.
-
Mucosal Application of a Low-Energy Electron Inactivated Respiratory Syncytial Virus Vaccine Shows Protective Efficacy in an Animal Model.Viruses. 2023 Aug 30;15(9):1846. doi: 10.3390/v15091846. Viruses. 2023. PMID: 37766253 Free PMC article.
-
Mucosal immunization with a low-energy electron inactivated respiratory syncytial virus vaccine protects mice without Th2 immune bias.Front Immunol. 2024 Apr 5;15:1382318. doi: 10.3389/fimmu.2024.1382318. eCollection 2024. Front Immunol. 2024. PMID: 38646538 Free PMC article.
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. . Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet (2010) 375:1545–55. doi: 10.1016/S0140-6736(10)60206-1 - DOI - PMC - PubMed
-
- Blunck BN, Aideyan L, Ye X, Avadhanula V, Ferlic-Stark L, Zechiedrich L, et al. . A prospective surveillance study on the kinetics of the humoral immune response to the respiratory syncytial virus fusion protein in adults in Houston, Texas. Vaccine (2021) 39:1248–56. doi: 10.1016/j.vaccine.2021.01.045 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

