Matrix Matters: Assessment of Commutability among BK Virus Assays and Standards
- PMID: 35997500
- PMCID: PMC9491175
- DOI: 10.1128/jcm.00555-22
Matrix Matters: Assessment of Commutability among BK Virus Assays and Standards
Abstract
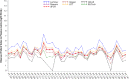
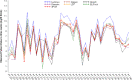
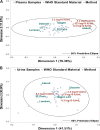
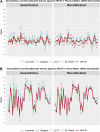
Quantitative testing of BK virus (BKPyV) nucleic acid has become the standard of care in transplant patients. While the relationship between interassay harmonization and commutability has been well characterized for other transplant-related viruses, it has been less well studied for BKPyV, particularly regarding differences in commutability between matrices. Here, interassay agreement was evaluated among six real-time nucleic acid amplification tests (NAATs) and one digital PCR (dPCR) BKPyV assay. Differences in the commutability of three quantitative standards was examined across all assays using a variety of statistical approaches. Panels, including 40 samples each of plasma and urine samples previously positive for BKPyV, together with one previously negative plasma sample and four previously negative urine samples, were tested using all assays, with each real-time NAAT utilizing its usual quantitative calibrators. Serial dilutions of WHO, National Institute for Standards and Technology (NIST), and commercially produced (Exact/Bio-Rad) reference materials were also run by each assay as unknowns. The agreement of the clinical sample values was assessed as a group and in a pairwise manner. The commutability was estimated using both relativistic and quantitative means. The quantitative agreement across assays in the urine samples was within a single log10 unit across all assays, while the results from the plasma samples varied by 2 to 3 log10 IU/mL. The commutability showed a similar disparity between the matrices. Recalibration using international standards diminished the resulting discrepancies in some but not all cases. Differences in the sample matrix can affect the commutability and interassay agreement of quantitative BKPyV assays. Differences in commutability between matrices may largely be due to factors other than those such as amplicon size, previously described as important in the case of cytomegalovirus. Continued efforts to standardize viral load measurements must address multiple sources of variability and account for differences in assay systems, quantitative standards, and sample matrices.
Keywords: BK virus; commutability; digital PCR; quantitative PCR; real-time PCR; standardization; viral load.
Conflict of interest statement
The authors declare a conflict of interest. R. T. Hayden has served on advisory boards for Roche Diagnostics, Quidel Corporation, Inflammatix, and T2 BioSciences; A. M. Caliendo has served on advisory boards for Cepheid, First Light, Visby, ID Connect, Quidel and Chromacode; B.A. Pinsky has received research funds from altona Diagnostics; S.K. Tan is currently an employee of and holds stock in Vir Biotechnology, Inc. J. Boonyaratanakornkit was an employee of Bio-Rad Laboratories/Exact Diagnostics. None of the other authors have potential conflicts of interest to disclose.
Figures
Similar articles
-
Impact of Fragmentation on Commutability of Epstein-Barr Virus and Cytomegalovirus Quantitative Standards.J Clin Microbiol. 2019 Dec 23;58(1):e00888-19. doi: 10.1128/JCM.00888-19. Print 2019 Dec 23. J Clin Microbiol. 2019. PMID: 31619529 Free PMC article.
-
Accuracy of quantitative viral secondary standards: a re-examination.J Clin Microbiol. 2024 Mar 13;62(3):e0166923. doi: 10.1128/jcm.01669-23. Epub 2024 Feb 21. J Clin Microbiol. 2024. PMID: 38380932 Free PMC article.
-
Commutability of the First World Health Organization International Standard for Human Cytomegalovirus.J Clin Microbiol. 2015 Oct;53(10):3325-33. doi: 10.1128/JCM.01495-15. Epub 2015 Aug 12. J Clin Microbiol. 2015. PMID: 26269622 Free PMC article.
-
Persistent Challenges of Interassay Variability in Transplant Viral Load Testing.J Clin Microbiol. 2020 Sep 22;58(10):e00782-20. doi: 10.1128/JCM.00782-20. Print 2020 Sep 22. J Clin Microbiol. 2020. PMID: 32554479 Free PMC article. Review.
-
Standardization of Nucleic Acid Tests for Clinical Measurements of Bacteria and Viruses.J Clin Microbiol. 2015 Jul;53(7):2008-14. doi: 10.1128/JCM.02136-14. Epub 2014 Nov 12. J Clin Microbiol. 2015. PMID: 25392365 Free PMC article. Review.
Cited by
-
Deep Sequencing and Molecular Characterisation of BK Virus and JC Virus WHO International Reference Materials for Clinical Diagnostic Use.Viruses. 2023 May 30;15(6):1289. doi: 10.3390/v15061289. Viruses. 2023. PMID: 37376589 Free PMC article.
References
-
- Hayden RT, Yan X, Wick MT, Rodriguez AB, Xiong X, Ginocchio CC, Mitchell MJ, Caliendo AM, College of American Pathologists Microbiology Resource Committee . 2012. Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. J Clin Microbiol 50:337–345. 10.1128/JCM.01287-11. - DOI - PMC - PubMed
-
- Pang XL, Fox JD, Fenton JM, Miller GG, Caliendo AM, Preiksaitis JK, American Society of Transplantation Infectious Diseases Community of Practice, Canadian Society of Transplantation . 2009. Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant 9:258–268. 10.1111/j.1600-6143.2008.02513.x. - DOI - PubMed
-
- Hayden RT, Hokanson KM, Pounds SB, Bankowski MJ, Belzer SW, Carr J, Diorio D, Forman MS, Joshi Y, Hillyard D, Hodinka RL, Nikiforova MN, Romain CA, Stevenson J, Valsamakis A, Balfour HH, Jr, U.S. EBV Working Group . 2008. Multicenter comparison of different real-time PCR assays for quantitative detection of Epstein-Barr virus. J Clin Microbiol 46:157–163. 10.1128/JCM.01252-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

