Role of Protein Damage Inflicted by Dopamine Metabolites in Parkinson's Disease: Evidence, Tools, and Outlook
- PMID: 35994383
- PMCID: PMC10225972
- DOI: 10.1021/acs.chemrestox.2c00193
Role of Protein Damage Inflicted by Dopamine Metabolites in Parkinson's Disease: Evidence, Tools, and Outlook
Abstract
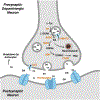
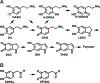
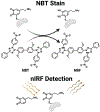
Dopamine is an important neurotransmitter that plays a critical role in motivational salience and motor coordination. However, dysregulated dopamine metabolism can result in the formation of reactive electrophilic metabolites which generate covalent adducts with proteins. Such protein damage can impair native protein function and lead to neurotoxicity, ultimately contributing to Parkinson's disease etiology. In this Review, the role of dopamine-induced protein damage in Parkinson's disease is discussed, highlighting the novel chemical tools utilized to drive this effort forward. Continued innovation of methodologies which enable detection, quantification, and functional response elucidation of dopamine-derived protein adducts is critical for advancing this field. Work in this area improves foundational knowledge of the molecular mechanisms that contribute to dopamine-mediated Parkinson's disease progression, potentially assisting with future development of therapeutic interventions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
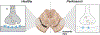
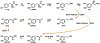
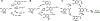
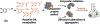
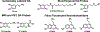

Similar articles
-
Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson's disease.Neurotox Res. 2003;5(3):165-76. doi: 10.1007/BF03033137. Neurotox Res. 2003. PMID: 12835121 Review.
-
Dysfunction of dopamine homeostasis: clues in the hunt for novel Parkinson's disease therapies.FASEB J. 2013 Jun;27(6):2101-10. doi: 10.1096/fj.12-226852. Epub 2013 Mar 5. FASEB J. 2013. PMID: 23463698 Review.
-
The therapeutic potential of neurotrophic factors in the treatment of Parkinson's disease.Exp Neurol. 1993 Nov;124(1):103-18. doi: 10.1006/exnr.1993.1181. Exp Neurol. 1993. PMID: 8282068 Review.
-
Polymorphism of the dopamine transporter type 1 gene modifies the treatment response in Parkinson's disease.Brain. 2015 May;138(Pt 5):1271-83. doi: 10.1093/brain/awv063. Epub 2015 Mar 23. Brain. 2015. PMID: 25805645 Free PMC article. Clinical Trial.
-
Dopamine restores cognitive motivation in Parkinson's disease.Brain. 2019 Mar 1;142(3):719-732. doi: 10.1093/brain/awy341. Brain. 2019. PMID: 30689734
Cited by
-
Orally Induced High Serum Level of Trimethylamine N-oxide Worsened Glial Reaction and Neuroinflammation on MPTP-Induced Acute Parkinson's Disease Model Mice.Mol Neurobiol. 2023 Sep;60(9):5137-5154. doi: 10.1007/s12035-023-03392-x. Epub 2023 Jun 2. Mol Neurobiol. 2023. PMID: 37266763
-
Iron Deposition in Parkinson's Disease: A Mini-Review.Cell Mol Neurobiol. 2024 Feb 23;44(1):26. doi: 10.1007/s10571-024-01459-4. Cell Mol Neurobiol. 2024. PMID: 38393383 Free PMC article. Review.
-
Investigation into Propolis Components Responsible for Inducing Skin Allergy: Air Oxidation of Caffeic Acid and Its Esters Contribute to Hapten Formation.Chem Res Toxicol. 2023 Jun 19;36(6):859-869. doi: 10.1021/acs.chemrestox.2c00386. Epub 2023 May 15. Chem Res Toxicol. 2023. PMID: 37184291 Free PMC article.
-
Salivary Biomarkers for Parkinson's Disease: A Systematic Review with Meta-Analysis.Cells. 2024 Feb 14;13(4):340. doi: 10.3390/cells13040340. Cells. 2024. PMID: 38391952 Free PMC article. Review.
-
Implantable Electrochemical Microsensors for In Vivo Monitoring of Animal Physiological Information.Nanomicro Lett. 2023 Dec 12;16(1):49. doi: 10.1007/s40820-023-01274-4. Nanomicro Lett. 2023. PMID: 38087121 Free PMC article. Review.
References
-
- Dorsey ER; Elbaz A; Nichols E; Abbasi N; Abd-Allah F; Abdelalim A; Adsuar JC; Ansha MG; Brayne C; Choi J-YJ; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17 (11), 939–953. DOI: 10.1016/s1474-4422(18)30295-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

