The Inactivated gE/ TK Gene - Deleted Vaccine Against Pseudorabies Virus Type II Confers Effective Protection in Mice and Pigs
- PMID: 35992698
- PMCID: PMC9389536
- DOI: 10.3389/fmicb.2022.943707
The Inactivated gE/ TK Gene - Deleted Vaccine Against Pseudorabies Virus Type II Confers Effective Protection in Mice and Pigs
Abstract
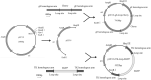
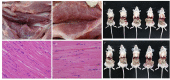
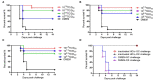
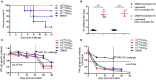
The highly virulent and antigenic variant of Pseudorabies virus (PRV) that emerged from classical Bartha-K61-vaccinated pig herds has caused substantial economic losses to the swine industry in China since 2011. A safe and more effective vaccine is most desirable. In this study, a gE/TK gene-deficient PRV, namely, HD/c, was constructed based on a PRV type II DX strain isolated from a commercial vaccine-immunized farm and the HD/c-based inactivated vaccine was formulated and evaluated for its safety, immunogenicity, and protective efficacy in mice and piglets. The resulting PRV HD/c strain has a similar growth curve to the parental DX strain. After vaccination, the inactivated HD/c vaccine did not cause any visible gross pathological or histopathological changes in the tissues of mice and piglets and provided rapid and potent protection against the challenge of the classical and variant PRVs at day 21 post-vaccination in mice. A single immunization of 108.5TCID50 inactivated PRV HD/c strain-elicited robust immunity with high titer of neutralizing antibody and provided complete protection from the lethal challenge of PRV DX strain in piglets. These results indicated that the inactivated PRV HD/c vaccine with the deletion of gE/TK genes was a safe and effective PRV vaccine candidate for the control of PRV.
Keywords: Pseudorabies virus type II; gE/TK deletion; inactivated vaccine; protection; safe.
Copyright © 2022 Jin, Yin, Xing, Huang, Fan, Fan, Qiu, Dong, Yan, Gu and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
An inactivated gE-deleted pseudorabies vaccine provides complete clinical protection and reduces virus shedding against challenge by a Chinese pseudorabies variant.BMC Vet Res. 2016 Dec 7;12(1):277. doi: 10.1186/s12917-016-0897-z. BMC Vet Res. 2016. PMID: 27923365 Free PMC article.
-
Safety and immunogenicity of an attenuated Chinese pseudorabies variant by dual deletion of TK&gE genes.BMC Vet Res. 2018 Sep 21;14(1):287. doi: 10.1186/s12917-018-1536-7. BMC Vet Res. 2018. PMID: 30241529 Free PMC article.
-
Better immune efficacy triggered by the inactivated gI/gE-deleted pseudorabies virus with the additional insertion of gC gene in mice and weaned pigs.Virus Res. 2021 Apr 15;296:198353. doi: 10.1016/j.virusres.2021.198353. Epub 2021 Feb 25. Virus Res. 2021. PMID: 33640358
-
Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine.Adv Vet Med. 1999;41:447-61. doi: 10.1016/s0065-3519(99)80034-2. Adv Vet Med. 1999. PMID: 9890035 Review.
-
Circumvention of maternal antibody interference by immunization of newborn pigs with glycoprotein gIII-deleted marker vaccine.Immunol Cell Biol. 1993 Oct;71 ( Pt 5):421-30. doi: 10.1038/icb.1993.48. Immunol Cell Biol. 1993. PMID: 8270271 Review.
Cited by
-
Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development.Vaccines (Basel). 2024 Sep 20;12(9):1078. doi: 10.3390/vaccines12091078. Vaccines (Basel). 2024. PMID: 39340108 Free PMC article. Review.
-
Generation and Characterization of Recombinant Pseudorabies Virus Delivering African Swine Fever Virus CD2v and p54.Int J Mol Sci. 2023 Dec 26;25(1):335. doi: 10.3390/ijms25010335. Int J Mol Sci. 2023. PMID: 38203508 Free PMC article.
-
Immunological characteristics of a recombinant alphaherpesvirus with an envelope-embedded Cap protein of circovirus.Front Immunol. 2024 Jul 16;15:1438371. doi: 10.3389/fimmu.2024.1438371. eCollection 2024. Front Immunol. 2024. PMID: 39081314 Free PMC article.
-
A Comprehensive Review of Our Understanding and Challenges of Viral Vaccines against Swine Pathogens.Viruses. 2024 May 24;16(6):833. doi: 10.3390/v16060833. Viruses. 2024. PMID: 38932126 Free PMC article. Review.
-
Development and immunogenicity evaluation of a quadruple-gene-deleted pseudorabies virus strain.Front Microbiol. 2024 Sep 20;15:1479794. doi: 10.3389/fmicb.2024.1479794. eCollection 2024. Front Microbiol. 2024. PMID: 39372271 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

