Revisiting the multisite phosphorylation that produces the M-phase supershift of key mitotic regulators
- PMID: 35976701
- PMCID: PMC9635296
- DOI: 10.1091/mbc.E22-04-0118
Revisiting the multisite phosphorylation that produces the M-phase supershift of key mitotic regulators
Abstract
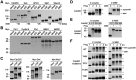
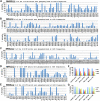
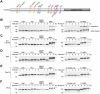
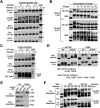
The term M-phase supershift denotes the phosphorylation-dependent substantial increase in the apparent molecular weight of numerous proteins of varied biological functions during M-phase induction. Although the M-phase supershift of multiple key mitotic regulators has been attributed to the multisite phosphorylation catalyzed by the Cdk1/cyclin B/Cks complex, this view is challenged by multiple lines of paradoxical observations. To solve this problem, we reconstituted the M-phase supershift of Xenopus Cdc25C, Myt1, Wee1A, APC3, and Greatwall in Xenopus egg extracts and characterized the supershift-producing phosphorylations. Our results demonstrate that their M-phase supershifts are each due to simultaneous phosphorylation of a considerable portion of S/T/Y residues in a long intrinsically disordered region that is enriched in both S/T residues and S/TP motifs. Although the major mitotic kinases in Xenopus egg extracts, Cdk1, MAPK, Plx1, and RSK2, are able to phosphorylate the five mitotic regulators, they are neither sufficient nor required to produce the M-phase supershift. Accordingly, inhibition of the four major mitotic kinase activities in Xenopus oocytes did not inhibit the M-phase supershift in okadaic acid-induced oocyte maturation. These findings indicate that the M-phase supershift is produced by a previously unrecognized category of mitotic phosphorylation that likely plays important roles in M-phase induction.
Figures








Similar articles
-
The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes.Mol Biol Cell. 2001 Jun;12(6):1791-9. doi: 10.1091/mbc.12.6.1791. Mol Biol Cell. 2001. PMID: 11408585 Free PMC article.
-
Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase- promoting complex at mitosis.Genes Dev. 1998 Aug 15;12(16):2549-59. doi: 10.1101/gad.12.16.2549. Genes Dev. 1998. PMID: 9716407 Free PMC article.
-
The xenopus Suc1/Cks protein promotes the phosphorylation of G(2)/M regulators.J Biol Chem. 1999 Dec 24;274(52):36839-42. doi: 10.1074/jbc.274.52.36839. J Biol Chem. 1999. PMID: 10601234
-
The activation of MAP kinase and p34cdc2/cyclin B during the meiotic maturation of Xenopus oocytes.Prog Cell Cycle Res. 2000;4:131-43. doi: 10.1007/978-1-4615-4253-7_12. Prog Cell Cycle Res. 2000. PMID: 10740821 Review.
-
Regulation of the meiotic cell cycle in oocytes.Curr Opin Cell Biol. 2000 Dec;12(6):666-75. doi: 10.1016/s0955-0674(00)00150-2. Curr Opin Cell Biol. 2000. PMID: 11063930 Review.
Cited by
-
Recombinant cyclin B-Cdk1-Suc1 capable of multi-site mitotic phosphorylation in vitro.PLoS One. 2024 Mar 25;19(3):e0299003. doi: 10.1371/journal.pone.0299003. eCollection 2024. PLoS One. 2024. PMID: 38527022 Free PMC article.
-
Cdc14B/Cyclin B1 signaling modulates the pathogenesis of sonic hedgehog subtype medulloblastoma.Am J Cancer Res. 2024 Jun 15;14(6):2868-2880. doi: 10.62347/CVAY8707. eCollection 2024. Am J Cancer Res. 2024. PMID: 39005661 Free PMC article.
References
-
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015). The cell cycle. In: Molecular Biology of the Cell, New York: Garland Science.
-
- Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, et al. (2011). Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal 4, ra42. - PMC - PubMed
-
- Bhatt RR, Ferrell JE Jr (2000). Cloning and characterization of Xenopus Rsk2, the predominant p90 Rsk isozyme in oocytes and eggs. J Biol Chem 275, 32983–32990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

