Herpes Simplex Virus Type 1 Preferentially Enhances Neuro-Inflammation and Senescence in Brainstem of Female Mice
- PMID: 35975996
- PMCID: PMC9472638
- DOI: 10.1128/jvi.01081-22
Herpes Simplex Virus Type 1 Preferentially Enhances Neuro-Inflammation and Senescence in Brainstem of Female Mice
Abstract
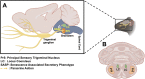
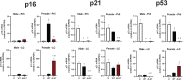
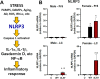
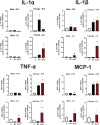
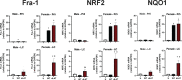
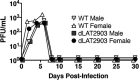
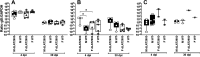
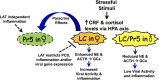
Following acute infection, herpes simplex virus 1 (HSV-1) establishes lifelong latency in neurons. The latency associated transcript (LAT) is the only viral gene abundantly expressed during latency. Wild-type (WT) HSV-1 reactivates more efficiently than LAT mutants because LAT promotes establishment and maintenance of latency. While sensory neurons in trigeminal ganglia (TG) are important sites for latency, brainstem is also a site for latency and reactivation from latency. The principal sensory nucleus of the spinal trigeminal tract (Pr5) likely harbors latent HSV-1 because it receives afferent inputs from TG. The locus coeruleus (LC), an adjacent brainstem region, sends axonal projections to cortical structures and is indirectly linked to Pr5. Senescent cells accumulate in the nervous system during aging and accelerate neurodegenerative processes. Generally senescent cells undergo irreversible cell cycle arrest and produce inflammatory cytokines and chemokines. Based on these observations, we hypothesized HSV-1 influences senescence and inflammation in Pr5 and LC of latently infected mice. This hypothesis was tested using a mouse model of infection. Strikingly, female but not age-matched male mice latently infected with a LAT null mutant (dLAT2903) exhibited significantly higher levels of senescence markers and inflammation in LC, including cell cycle inhibitor p16, NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3), IL-1α, and IL-β. Conversely, Pr5 in female but not male mice latently infected with WT HSV-1 or dLAT2903 exhibited enhanced expression of important inflammatory markers. The predilection of HSV-1 to induce senescence and inflammation in key brainstem regions of female mice infers that enhanced neurodegeneration occurs. IMPORTANCE HSV-1 (herpes simplex virus 1), an important human pathogen, establishes lifelong latency in neurons in trigeminal ganglia and the central nervous system. In contrast to productive infection, the only viral transcript abundantly expressed in latently infected neurons is the latency associated transcript (LAT). The brainstem, including principal sensory nucleus of the spinal trigeminal tract (Pr5) and locus coeruleus (LC), may expedite HSV-1 spread from trigeminal ganglia to the brain. Enhanced senescence and expression of key inflammatory markers were detected in LC of female mice latently infected with a LAT null mutant (dLAT2903) relative to age-matched male or female mice latently infected with wild-type HSV-1. Conversely, wild-type HSV-1 and dLAT2903 induced higher levels of senescence and inflammatory markers in Pr5 of latently infected female mice. In summary, enhanced inflammation and senescence in LC and Pr5 of female mice latently infected with HSV-1 are predicted to accelerate neurodegeneration.
Keywords: HSV-1; LAT; brainstem; immune senescence; inflammation; latency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Examination of neuro-inflammation and senescence in brainstem of aged mice latently infected with human alphaherpesvirus 1 (HSV-1).Virus Res. 2024 Sep;347:199420. doi: 10.1016/j.virusres.2024.199420. Epub 2024 Jun 21. Virus Res. 2024. PMID: 38880336 Free PMC article.
-
The persistent elevated cytokine mRNA levels in trigeminal ganglia of mice latently infected with HSV-1 are not due to the presence of latency associated transcript (LAT) RNAs.Virus Res. 1998 Mar;54(1):1-8. doi: 10.1016/s0168-1702(98)00007-0. Virus Res. 1998. PMID: 9660066
-
The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal Ganglia of acutely infected mice.J Virol. 2005 May;79(10):6162-71. doi: 10.1128/JVI.79.10.6162-6171.2005. J Virol. 2005. PMID: 15858001 Free PMC article.
-
Bovine Herpes Virus 1 (BHV-1) and Herpes Simplex Virus Type 1 (HSV-1) Promote Survival of Latently Infected Sensory Neurons, in Part by Inhibiting Apoptosis.J Cell Death. 2013 Apr 9;6:1-16. doi: 10.4137/JCD.S10803. eCollection 2013. J Cell Death. 2013. PMID: 25278776 Free PMC article. Review.
-
Herpes simplex virus type 1 and bovine herpesvirus 1 latency.Clin Microbiol Rev. 2003 Jan;16(1):79-95. doi: 10.1128/CMR.16.1.79-95.2003. Clin Microbiol Rev. 2003. PMID: 12525426 Free PMC article. Review.
Cited by
-
Glucocorticoid receptor and specificity protein 1 (Sp1) or Sp3, but not the antibiotic Mithramycin A, stimulates human alphaherpesvirus 1 (HSV-1) replication.Antiviral Res. 2024 May;225:105870. doi: 10.1016/j.antiviral.2024.105870. Epub 2024 Mar 29. Antiviral Res. 2024. PMID: 38556059
-
Contribution of viral and bacterial infections to senescence and immunosenescence.Front Cell Infect Microbiol. 2023 Sep 11;13:1229098. doi: 10.3389/fcimb.2023.1229098. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37753486 Free PMC article. Review.
-
Disease parameters following ocular herpes simplex virus type 1 infection are similar in male and female BALB/C mice.PLoS One. 2023 Jun 15;18(6):e0287194. doi: 10.1371/journal.pone.0287194. eCollection 2023. PLoS One. 2023. PMID: 37319284 Free PMC article.
-
Olfactory and trigeminal routes of HSV-1 CNS infection with regional microglial heterogeneity.J Virol. 2024 Nov 19;98(11):e0096824. doi: 10.1128/jvi.00968-24. Epub 2024 Oct 30. J Virol. 2024. PMID: 39475273
-
Asymptomatic herpes simplex virus brain infection elicits cellular senescence phenotypes in the central nervous system of mice suffering multiple sclerosis-like disease.Commun Biol. 2024 Jul 4;7(1):811. doi: 10.1038/s42003-024-06486-x. Commun Biol. 2024. PMID: 38965360 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

