Reduced Expression of TMEM16A Impairs Nitric Oxide-Dependent Cl- Transport in Retinal Amacrine Cells
- PMID: 35966201
- PMCID: PMC9363626
- DOI: 10.3389/fncel.2022.937060
Reduced Expression of TMEM16A Impairs Nitric Oxide-Dependent Cl- Transport in Retinal Amacrine Cells
Abstract
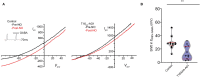
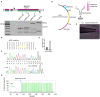
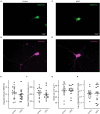
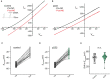
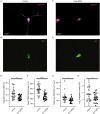
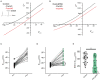
Postsynaptic cytosolic Cl- concentration determines whether GABAergic and glycinergic synapses are inhibitory or excitatory. We have shown that nitric oxide (NO) initiates the release of Cl- from acidic internal stores into the cytosol of retinal amacrine cells (ACs) thereby elevating cytosolic Cl-. In addition, we found that cystic fibrosis transmembrane conductance regulator (CFTR) expression and Ca2+ elevations are necessary for the transient effects of NO on cytosolic Cl- levels, but the mechanism remains to be elucidated. Here, we investigated the involvement of TMEM16A as a possible link between Ca2+ elevations and cytosolic Cl- release. TMEM16A is a Ca2+-activated Cl- channel that is functionally coupled with CFTR in epithelia. Both proteins are also expressed in neurons. Based on this and its Ca2+ dependence, we test the hypothesis that TMEM16A participates in the NO-dependent elevation in cytosolic Cl- in ACs. Chick retina ACs express TMEM16A as shown by Western blot analysis, single-cell PCR, and immunocytochemistry. Electrophysiology experiments demonstrate that TMEM16A functions in amacrine cells. Pharmacological inhibition of TMEM16A with T16inh-AO1 reduces the NO-dependent Cl- release as indicated by the diminished shift in the reversal potential of GABAA receptor-mediated currents. We confirmed the involvement of TMEM16A in the NO-dependent Cl- release using CRISPR/Cas9 knockdown of TMEM16A. Two different modalities targeting the gene for TMEM16A (ANO1) were tested in retinal amacrine cells: an all-in-one plasmid vector and crRNA/tracrRNA/Cas9 ribonucleoprotein. The all-in-one CRISPR/Cas9 modality did not change the expression of TMEM16A protein and produced no change in the response to NO. However, TMEM16A-specific crRNA/tracrRNA/Cas9 ribonucleoprotein effectively reduces both TMEM16A protein levels and the NO-dependent shift in the reversal potential of GABA-gated currents. These results show that TMEM16A plays a role in the NO-dependent Cl- release from retinal ACs.
Keywords: CRISPR/Cas9; TMEM16/anoctamin; amacrine cell; intracellular Cl−; nitric oxide (NO); retina.
Copyright © 2022 Rodriguez, Zhong, Simpson and Gleason.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
A role for the cystic fibrosis transmembrane conductance regulator in the nitric oxide-dependent release of Cl- from acidic organelles in amacrine cells.J Neurophysiol. 2017 Nov 1;118(5):2842-2852. doi: 10.1152/jn.00511.2017. Epub 2017 Aug 23. J Neurophysiol. 2017. PMID: 28835528 Free PMC article.
-
Adenylate Cyclase 1 Links Calcium Signaling to CFTR-Dependent Cytosolic Chloride Elevations in Chick Amacrine Cells.Front Cell Neurosci. 2021 Aug 11;15:726605. doi: 10.3389/fncel.2021.726605. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34456687 Free PMC article.
-
Inhibition of endocytosis suppresses the nitric oxide-dependent release of Cl- in retinal amacrine cells.PLoS One. 2018 Jul 25;13(7):e0201184. doi: 10.1371/journal.pone.0201184. eCollection 2018. PLoS One. 2018. PMID: 30044876 Free PMC article.
-
Bestrophin and TMEM16-Ca(2+) activated Cl(-) channels with different functions.Cell Calcium. 2009 Oct;46(4):233-41. doi: 10.1016/j.ceca.2009.09.003. Epub 2009 Sep 26. Cell Calcium. 2009. PMID: 19783045 Review.
-
TMEM16A (ANO1) as a therapeutic target in cystic fibrosis.Curr Opin Pharmacol. 2022 Jun;64:102206. doi: 10.1016/j.coph.2022.102206. Epub 2022 Mar 29. Curr Opin Pharmacol. 2022. PMID: 35364521 Review.
Cited by
-
Cholecystokinin receptor type A are involved in the circadian rhythm of the mouse retina.Heliyon. 2024 Jun 7;10(12):e32653. doi: 10.1016/j.heliyon.2024.e32653. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39183886 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

