Dual effects of supplemental oxygen on pulmonary infection, inflammatory lung injury, and neuromodulation in aging and COVID-19
- PMID: 35964839
- PMCID: PMC9367207
- DOI: 10.1016/j.freeradbiomed.2022.08.004
Dual effects of supplemental oxygen on pulmonary infection, inflammatory lung injury, and neuromodulation in aging and COVID-19
Abstract
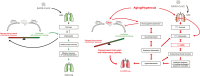
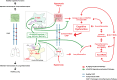
Clinical studies have shown a significant positive correlation between age and the likelihood of being infected with SARS-CoV-2. This increased susceptibility is positively correlated with chronic inflammation and compromised neurocognitive functions. Postmortem analyses suggest that acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), with systemic and lung hyperinflammation, can cause significant morbidity and mortality in COVID-19 patients. Supraphysiological supplemental oxygen, also known as hyperoxia, is commonly used to treat decreased blood oxygen saturation in COVID-19 patients. However, prolonged exposure to hyperoxia alone can cause oxygen toxicity, due to an excessive increase in the levels of reactive oxygen species (ROS), which can overwhelm the cellular antioxidant capacity. Subsequently, this causes oxidative cellular damage and increased levels of aging biomarkers, such as telomere shortening and inflammaging. The oxidative stress in the lungs and brain can compromise innate immunity, resulting in an increased susceptibility to secondary lung infections, impaired neurocognitive functions, and dysregulated hyperinflammation, which can lead to ALI/ARDS, and even death. Studies indicate that lung inflammation is regulated by the central nervous system, notably, the cholinergic anti-inflammatory pathway (CAIP), which is innervated by the vagus nerve and α7 nicotinic acetylcholine receptors (α7nAChRs) on lung cells, particularly lung macrophages. The activation of α7nAChRs attenuates oxygen toxicity in the lungs and improves clinical outcomes by restoring hyperoxia-compromised innate immunity. Mechanistically, α7nAChR agonist (e.g., GAT 107 and GTS-21) can regulate redox signaling by 1) activating Nrf2, a master regulator of the antioxidant response and a cytoprotective defense system, which can decrease cellular damage caused by ROS and 2) inhibiting the activation of the NF-κB-mediated inflammatory response. Notably, GTS-21 has been shown to be safe and it improves neurocognitive functions in humans. Therefore, targeting the α7nAChR may represent a viable therapeutic approach for attenuating dysregulated hyperinflammation-mediated ARDS and sepsis in COVID-19 patients receiving prolonged oxygen therapy.
Keywords: Acute respiratory distress syndrome (ARDS); Aging; COVID-19; Cholinergic anti-inflammatory pathway; Inflammation; Mitochondria; Supplemental oxygen therapy; α7 nicotinic acetylcholine receptor.
Copyright © 2022. Published by Elsevier Inc.
Figures


Similar articles
-
GAT107-mediated α7 nicotinic acetylcholine receptor signaling attenuates inflammatory lung injury and mortality in a mouse model of ventilator-associated pneumonia by alleviating macrophage mitochondrial oxidative stress via reducing MnSOD-S-glutathionylation.Redox Biol. 2023 Apr;60:102614. doi: 10.1016/j.redox.2023.102614. Epub 2023 Jan 20. Redox Biol. 2023. PMID: 36717349 Free PMC article.
-
The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function.Mol Med. 2014 Jun 19;20(1):238-47. doi: 10.2119/molmed.2013.00086. Mol Med. 2014. Retraction in: Mol Med. 2020 Dec 30;26(1):132. doi: 10.1186/s10020-020-00265-0 PMID: 24664237 Free PMC article. Retracted.
-
The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function.Mol Med. 2020 Oct 30;26(1):98. doi: 10.1186/s10020-020-00224-9. Mol Med. 2020. PMID: 33126860 Free PMC article.
-
Alveolar Hyperoxia and Exacerbation of Lung Injury in Critically Ill SARS-CoV-2 Pneumonia.Med Sci (Basel). 2023 Nov 1;11(4):70. doi: 10.3390/medsci11040070. Med Sci (Basel). 2023. PMID: 37987325 Free PMC article. Review.
-
Innate Receptor Activation Patterns Involving TLR and NLR Synergisms in COVID-19, ALI/ARDS and Sepsis Cytokine Storms: A Review and Model Making Novel Predictions and Therapeutic Suggestions.Int J Mol Sci. 2021 Feb 20;22(4):2108. doi: 10.3390/ijms22042108. Int J Mol Sci. 2021. PMID: 33672738 Free PMC article. Review.
Cited by
-
GAT107-mediated α7 nicotinic acetylcholine receptor signaling attenuates inflammatory lung injury and mortality in a mouse model of ventilator-associated pneumonia by alleviating macrophage mitochondrial oxidative stress via reducing MnSOD-S-glutathionylation.Redox Biol. 2023 Apr;60:102614. doi: 10.1016/j.redox.2023.102614. Epub 2023 Jan 20. Redox Biol. 2023. PMID: 36717349 Free PMC article.
-
Identification of berberine as a potential therapeutic strategy for kidney clear cell carcinoma and COVID-19 based on analysis of large-scale datasets.Front Immunol. 2023 Mar 23;14:1038651. doi: 10.3389/fimmu.2023.1038651. eCollection 2023. Front Immunol. 2023. PMID: 37033923 Free PMC article.
-
Acute lung injury caused by sepsis: how does it happen?Front Med (Lausanne). 2023 Nov 21;10:1289194. doi: 10.3389/fmed.2023.1289194. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38076268 Free PMC article. Review.
-
Mitochondria in COVID-19: from cellular and molecular perspective.Front Physiol. 2024 Jun 21;15:1406635. doi: 10.3389/fphys.2024.1406635. eCollection 2024. Front Physiol. 2024. PMID: 38974521 Free PMC article. Review.
-
Sequential respiratory support in septic patients undergoing continuous renal replacement therapy: A study based on MIMIC-III database.Heliyon. 2024 Mar 16;10(6):e27563. doi: 10.1016/j.heliyon.2024.e27563. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524548 Free PMC article.
References
-
- Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. - DOI - PMC - PubMed
-
- Lai X., Wang M., Qin C., Tan L., Ran L., Chen D., Zhang H., Shang K., Xia C., Wang S., Xu S., Wang W. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in wuhan, China. JAMA netw. Open. 2020;3:e209666. doi: 10.1001/jamanetworkopen.2020.9666. - DOI - PMC - PubMed
-
- Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O'Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. - DOI - PMC - PubMed
-
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

