Long-Term Strenuous Exercise Promotes Vascular Injury by Selectively Damaging the Tunica Media: Experimental Evidence
- PMID: 35958697
- PMCID: PMC9357576
- DOI: 10.1016/j.jacbts.2022.02.017
Long-Term Strenuous Exercise Promotes Vascular Injury by Selectively Damaging the Tunica Media: Experimental Evidence
Abstract
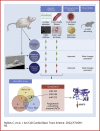
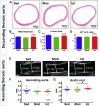
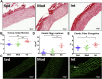
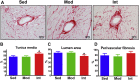
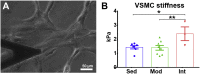
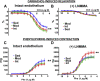
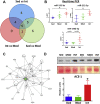
Moderate exercise has well-founded benefits in cardiovascular health. However, increasing, yet controversial, evidence suggests that extremely trained athletes may not be protected from cardiovascular events as much as moderately trained individuals. In our rodent model, intensive but not moderate training promoted aorta and carotid stiffening and elastic lamina ruptures, tunica media thickening of intramyocardial arteries, and an imbalance between vasoconstrictor and relaxation agents. An up-regulation of angiotensin-converter enzyme, miR-212, miR-132, and miR-146b might account for this deleterious remodeling. Most changes remained after a 4-week detraining. In conclusion, our results suggest that intensive training blunts the benefits of moderate exercise.
Keywords: CACS, coronary artery calcium score; CAD, coronary artery disease; CV, cardiovascular; MMP9, matrix metalloproteinase 9; NO, nitric oxide; Phe, phenylephrine; VSMC, vascular smooth muscle cell; atherosclerosis; coronary artery disease; endurance exercise; extreme sport; vascular stiffening.
© 2022 The Authors.
Conflict of interest statement
This work was partially supported by grants from the Instituto de Salud Carlos III (PI13/01580, PI16/00703, PI19/00443), co-funded by the European Union; CERCA program/Generalitat de Catalunya; CIBERCV (16/11/00354); and Spanish Ministry of Economy and Competitiveness (DPI2017-83721-P). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Similar articles
-
Salusin-β Promotes Vascular Smooth Muscle Cell Migration and Intimal Hyperplasia After Vascular Injury via ROS/NFκB/MMP-9 Pathway.Antioxid Redox Signal. 2016 Jun 20;24(18):1045-57. doi: 10.1089/ars.2015.6475. Epub 2016 Apr 8. Antioxid Redox Signal. 2016. PMID: 26952533
-
Exercise training and vascular cell phenotype in a swine model of familial hypercholesterolaemia: conduit arteries and veins.Exp Physiol. 2014 Feb;99(2):454-65. doi: 10.1113/expphysiol.2013.075838. Epub 2013 Nov 8. Exp Physiol. 2014. PMID: 24213857 Free PMC article.
-
Cyclosporine up-regulates Krüppel-like factor-4 (KLF4) in vascular smooth muscle cells and drives phenotypic modulation in vivo.J Pharmacol Exp Ther. 2010 Apr;333(1):34-42. doi: 10.1124/jpet.109.163949. Epub 2010 Jan 20. J Pharmacol Exp Ther. 2010. PMID: 20089806 Free PMC article.
-
The "Extreme Exercise Hypothesis": Recent Findings and Cardiovascular Health Implications.Curr Treat Options Cardiovasc Med. 2018 Aug 28;20(10):84. doi: 10.1007/s11936-018-0674-3. Curr Treat Options Cardiovasc Med. 2018. PMID: 30155804 Free PMC article. Review.
-
Regulation of coronary blood flow during exercise.Physiol Rev. 2008 Jul;88(3):1009-86. doi: 10.1152/physrev.00045.2006. Physiol Rev. 2008. PMID: 18626066 Review.
Cited by
-
Lifelong endurance exercise and its relation with coronary atherosclerosis.Eur Heart J. 2023 Jul 7;44(26):2388-2399. doi: 10.1093/eurheartj/ehad152. Eur Heart J. 2023. PMID: 36881712 Free PMC article.
-
Moderate-Intensity and High-Intensity Interval Exercise Training Offer Equal Cardioprotection, with Different Mechanisms, during the Development of Type 2 Diabetes in Rats.Nutrients. 2024 Jan 31;16(3):431. doi: 10.3390/nu16030431. Nutrients. 2024. PMID: 38337716 Free PMC article.
-
Something is moving in sports-related sudden cardiac death … is it time to change our minds?Europace. 2023 Feb 16;25(2):255-257. doi: 10.1093/europace/euac274. Europace. 2023. PMID: 36635946 Free PMC article. No abstract available.
-
Intense long-term training impairs brain health compared with moderate exercise: Experimental evidence and mechanisms.Ann N Y Acad Sci. 2022 Dec;1518(1):282-298. doi: 10.1111/nyas.14912. Epub 2022 Oct 18. Ann N Y Acad Sci. 2022. PMID: 36256544 Free PMC article.
-
Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice.Antioxidants (Basel). 2022 Sep 23;11(10):1891. doi: 10.3390/antiox11101891. Antioxidants (Basel). 2022. PMID: 36290614 Free PMC article.
References
-
- Guasch E., Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol. 2017;14:88–101. - PubMed
-
- Möhlenkamp S., Lehmann N., Breuckmann F., et al. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–1910. - PubMed
-
- Armstrong M.E.G., Green J., Reeves G.K., Beral V., Cairns B.J., Million Women Study Collaborators Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. - PubMed
-
- Roberts W.O., Schwartz R.S., Kraus S.M., et al. Long-term marathon running is associated with low coronary plaque formation in women. Med Sci Sports Exerc. 2017;49:641–645. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

